Impact of hepatitis B and hepatitis C virus infections in a hematology-oncology unit at a children's hospital in Nicaragua, 1997 to 1999
- PMID: 11986270
- PMCID: PMC119972
- DOI: 10.1128/cdli.9.3.622-626.2002
Impact of hepatitis B and hepatitis C virus infections in a hematology-oncology unit at a children's hospital in Nicaragua, 1997 to 1999
Abstract
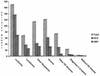
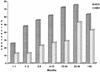
The risk of acquiring both hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in patients with hematological-oncological disorders has been documented. However, the impact and risk factors for such infections from different geographical areas vary, and the use of both immunological and molecular assays to determine HCV infections has been our approach. Children from a hematology-oncology unit (HOU) in Nicaragua were studied for both HBV and HCV serological markers; studies for the latter used both immunological (anti-HCV) and molecular (HCV RNA) assays. The children from the HOU included patients with leukemia, lymphoma, other neoplasias, and anemia and a smaller group with other hematological diseases. As a control group, children from other units at the same hospital were enrolled, as well as health care workers attending both patient populations. Pertinent clinical and personal data for each child at the HOU were obtained for statistical analysis. Of the 625 children from the HOU enrolled in this study 53.3% were infected with HCV and 29.4% had a prior or present HBV infection. In the child patient control group 3.2% had HBV markers and all were negative for HCV. The group of children with leukemia had the highest infection rate for both HBV and HCV. However, the determination of anti-HCV was found to have an overall low sensitivity in children from HOU, and a retest consisting of a molecular assay to determine HCV RNA was performed to better establish the total number of HCV-infected subjects in this group. The highest independent risk factor for infection was hospitalization. The very high prevalence rates for both HBV and HCV infection in this patient group indicate an urgent need to implement better control of known risk factors and to consider the use of both immunological and molecular assays for HCV diagnostic purposes.
Figures
References
-
- Allander, T., A. Gruber, M. Naghavi, A. Beyene, T. Söderström, M. Björkholm, L. Grillner, and M. A. A. Persson. 1995. Frequent patient-to-patient transmission of hepatitis C virus in a haematology ward. Lancet 345:603-606. - PubMed
-
- Arauz, R. P., H. Norder, K. A. Visona, and O. L. Magnius. 1997. Genotype F prevails in HBV infected patients of Hispanic origin in Central America and may carry the precore stop mutant. J. Med. Virol. 51:305-312. - PubMed
-
- Damen, M., H. L. Zaaijer, H. T. M. Cuypers, H. Vrielink, C. L. van der Poel, H. W. Reesink, and P. N. Lelie. 1995. Reliability of the third-generation recombinant immunoblot assay for hepatitis C virus. Transfusion 35:745-749. - PubMed
-
- De Rosa, G., M. L. Gobbo, A. De Renzo, R. Notaro, S. Garofalo, M. Grimaldi, A. Apuzzo, F. Chiurazzi, M. Picardi, M. Matarazzo, and B. Rotoli. 1997. High prevalence of hepatitis C virus infection in patients with B-cell lymphoproliferative disorders in Italy. Am. J. Hematol. 55:77-82. - PubMed
-
- Dibenedetto, S. P., V. Miraglia, A. M. Ippolito, S. D'Amico, L. Lo Nigro, R. Ragusa, and G. Schiliro. 1996. Reduction in the incidence of infection by hepatitis C virus in children with acute lymphoblastic leukemia after suspension of sampling from the finger. Brief reports. Pediatr. Infect. Dis. J. 15:265-266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials