Spike-independent release of ATP from Xenopus spinal neurons evoked by activation of glutamate receptors
- PMID: 11986374
- PMCID: PMC2290290
- DOI: 10.1113/jphysiol.2001.013193
Spike-independent release of ATP from Xenopus spinal neurons evoked by activation of glutamate receptors
Abstract
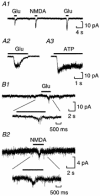
As the release of ATP from neurons has only been directly studied in a few cases, we have used patch sniffing to examine ATP release from Xenopus spinal neurons. ATP release was detected following intracellular current injection to evoke spikes. However, spiking was not essential as both glutamate and NMDA could evoke release of ATP in the presence of TTX. Neither acetylcholine nor high K(+) was effective at inducing ATP release in the presence of TTX. Although Cd(2+) blocked glutamate-evoked release of ATP suggesting a dependence on Ca(2+) entry, neither omega-conotoxin-GVIA nor nifedipine prevented ATP release. N-type and L-type channels are thus not essential for glutamate-evoked ATP release. That glutamate receptors can elicit release in the absence of spiking suggests a close physical relationship between these receptors, the Ca(2+) channels and release sites. As the dependence of ATP release on the influx of Ca(2+) through Ca(2+) channel subtypes differs from that of synaptic transmitter release, ATP may be released from sites that are distinct from those of the principal transmitter. In addition to its role as a fast transmitter, ATP may thus be released as a consequence of the activation of excitatory glutamatergic synapses and act to signal information about activity patterns in the nervous system.
Figures






References
-
- Anderson CM, Baldwin SA, Young JD, Cass CE, Parkinson FE. Distribution of mRNA encoding a nitrobenzylthioinosine-insensitive nucleoside transporter (ENT2) in rat brain. Brain Research. Molecular Brain Research. 1999a;70:293–297. - PubMed
-
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, Parkinson FE. Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. Journal of Neurochemistry. 1999b;73:867–873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

