Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus
- PMID: 11991960
- PMCID: PMC137021
- DOI: 10.1128/jvi.76.11.5315-5325.2002
Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus
Abstract
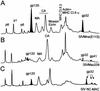
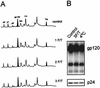
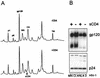
Human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) particles typically contain small amounts of the surface envelope protein (SU), and this is widely believed to be due to shedding of SU from mature virions. We purified proteins from HIV-1 and SIV isolates using procedures which allow quantitative measurements of viral protein content and determination of the ratios of gag- and env-encoded proteins in virions. All of the HIV-1 and most of the SIV isolates examined contained low levels of envelope proteins, with Gag:Env ratios of approximately 60:1. Based on an estimate of 1,200 to 2,500 Gag molecules per virion, this corresponds to an average of between 21 and 42 SU molecules, or between 7 and 14 trimers, per particle. In contrast, some SIV isolates contained levels of SU at least 10-fold greater than SU from HIV-1 isolates. Quantification of relative amounts of SU and transmembrane envelope protein (TM) provides a means to assess the impact of SU shedding on virion SU content, since such shedding would be expected to result in a molar excess of TM over SU on virions that had shed SU. With one exception, viruses with sufficient SU and TM to allow quantification were found to have approximately equivalent molar amounts of SU and TM. The quantity of SU associated with virions and the SU:TM ratios were not significantly changed during multiple freeze-thaw cycles or purification through sucrose gradients. Exposure of purified HIV-1 and SIV to temperatures of 55 degrees C or greater for 1 h resulted in loss of most of the SU from the virus but retention of TM. Incubation of purified virus with soluble CD4 at 37 degrees C resulted in no appreciable loss of SU from either SIV or HIV-1. These results indicate that the association of SU and TM on the purified virions studied is quite stable. These findings suggest that incorporation of SU-TM complexes into the viral membrane may be the primary factor determining the quantity of SU associated with SIV and HIV-1 virions, rather than shedding of SU from mature virions.
Figures


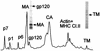



References
-
- Allan, J. S., E. M. Whitehead, K. Strout, M. Short, P. Kanda, T. K. Hart, and P. J. Bugelski. 1992. Strong association of simian immunodeficiency virus (SIVagm) envelope glycoprotein heterodimers: possible role in receptor-mediated activation. AIDS Res. Hum. Retrovir. 8:2011-2020. - PubMed
-
- Arthur, L. O., J. W. Bess, E. N. Chertova, J. L. Rossio, M. T. Esser, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retrovir. 14(Suppl. 3):S311-S319. - PubMed
-
- Benveniste, R. E., R. W. Hill, L. J. Eron, U. M. Csaikl, W. B. Knott, L. E. Henderson, R. C. Sowder, K. Nagashima, and M. A. Gonda. 1990. Characterization of clones of HIV-1-infected HuT 78 cells defective in gag gene processing and of SIV clones producing large amounts of envelope glycoprotein. J. Med. Primatol. 19:351-366. - PubMed
-
- Bess, J. W., Jr., R. J. Gorelick, W. J. Bosche, L. E. Henderson, and L. O. Arthur. 1997. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology 230:134-144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

