Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization
- PMID: 11991964
- PMCID: PMC137015
- DOI: 10.1128/jvi.76.11.5357-5368.2002
Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization
Abstract
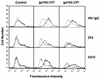
In this study, we have investigated the effect of specific mutations in human immunodeficiency virus type 1 (HIV-1) envelope (Env) on antibody production in an effort to improve humoral immune responses to this glycoprotein by DNA vaccination. Mice were injected with plasmid expression vectors encoding HIV Env with modifications in regions that might affect this response. Elimination of conserved glycosylation sites did not substantially enhance humoral or cytotoxic-T-lymphocyte (CTL) immunity. In contrast, a modified gp140 with different COOH-terminal mutations intended to mimic a fusion intermediate and stabilize trimer formation enhanced humoral immunity without reducing the efficacy of the CTL response. This mutant, with deletions in the cleavage site, fusogenic domain, and spacing of heptad repeats 1 and 2, retained native antigenic conformational determinants as defined by binding to known monoclonal antibodies or CD4, oligomer formation, and virus neutralization in vitro. Importantly, this modified Env, gp140 Delta CFI, stimulated the antibody response to native gp160 while it retained its ability to induce a CTL response, a desirable feature for an AIDS vaccine.
Figures





References
-
- Binley, J., and J. P. Moore. 1997. HIV-cell fusion. The viral mousetrap. Nature 387:346-348. (Erratum, 389:131.) - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
-
- Binley, J. M., R. Wyatt, E. Desjardins, P. D. Kwong, W. Hendrickson, J. P. Moore, and J. Sodroski. 1998. Analysis of the interaction of antibodies with a conserved enzymatically deglycosylated core of the HIV type 1 envelope glycoprotein 120. AIDS Res. Hum. Retrovir. 14:191-198. - PubMed
-
- Bolognesi, D. P., and T. J. Matthews. 1998. HIV vaccines. Viral envelope fails to deliver? Nature 391:638-639. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

