In vitro selection and characterization of influenza A (A/N9) virus variants resistant to a novel neuraminidase inhibitor, A-315675
- PMID: 11991966
- PMCID: PMC137025
- DOI: 10.1128/jvi.76.11.5380-5386.2002
In vitro selection and characterization of influenza A (A/N9) virus variants resistant to a novel neuraminidase inhibitor, A-315675
Abstract
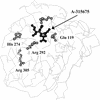
With the recent introduction of neuraminidase (NA) inhibitors into clinical practice for the treatment of influenza virus infections, considerable attention has been focused on the potential for resistance development and cross-resistance between different agents from this class. A-315675 is a novel influenza virus NA inhibitor that has potent enzyme activity and is highly active in cell culture against a variety of strains of influenza A and B viruses. To further assess the therapeutic potential of this compound, in vitro resistance studies have been conducted and a comparative assessment has been made relative to oseltamivir carboxylate. The development of viral resistance to A-315675 was studied by in vitro serial passage of influenza A/N9 virus strains grown in MDCK cells in the presence of increasing concentrations of A-315675. Parallel passaging experiments were conducted with oseltamivir carboxylate, the active form of a currently marketed oral agent for the treatment of influenza virus infections. Passage experiments with A-315675 identified a variant at passage 8 that was 60-fold less susceptible to the compound. Sequencing of the viral population identified an E119D mutation in the NA gene, but no mutations were observed in the hemagglutinin (HA) gene. However, by passage 10 (2.56 microM A-315675), two mutations (R233K, S339P) in the HA gene appeared in addition to the E119D mutation in the NA gene, resulting in a 310-fold-lower susceptibility to A-315675. Further passaging at higher drug concentrations had no effect on the generation of further NA or HA mutations (20.5 microM A-315675). This P15 virus displayed 355-fold-lower susceptibility to A-315675 and >175-fold-lower susceptibility to zanamivir than did wild-type virus, but it retained a high degree of susceptibility to oseltamivir carboxylate. By comparison, virus variants recovered from passaging against oseltamivir carboxylate (passage 14) harbored an E119V mutation and displayed a 6,000-fold-lower susceptibility to oseltamivir carboxylate and a 175-fold-lower susceptibility to zanamivir than did wild-type virus. Interestingly, this mutant still retained susceptibility to A-315675 (42-fold loss). This suggests that cross-resistance between A-315675- and oseltamivir carboxylate-selected variants in vitro is minimal.
Figures
Similar articles
-
Phenotypic drug susceptibility assay for influenza virus neuraminidase inhibitors.Clin Diagn Lab Immunol. 2004 Jan;11(1):21-8. doi: 10.1128/cdli.11.1.21-28.2004. Clin Diagn Lab Immunol. 2004. PMID: 14715540 Free PMC article.
-
Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants.Antimicrob Agents Chemother. 2001 Dec;45(12):3403-8. doi: 10.1128/AAC.45.12.3403-3408.2001. Antimicrob Agents Chemother. 2001. PMID: 11709315 Free PMC article.
-
Mutations conferring zanamivir resistance in human influenza virus N2 neuraminidases compromise virus fitness and are not stably maintained in vitro.J Antimicrob Chemother. 2006 Oct;58(4):723-32. doi: 10.1093/jac/dkl321. Epub 2006 Aug 5. J Antimicrob Chemother. 2006. PMID: 16891631
-
Recovery of drug-resistant influenza virus from immunocompromised patients: a case series.J Infect Dis. 2006 Mar 15;193(6):760-4. doi: 10.1086/500465. Epub 2006 Feb 13. J Infect Dis. 2006. PMID: 16479508 Review.
-
Treatment of influenza with neuraminidase inhibitors: virological implications.Philos Trans R Soc Lond B Biol Sci. 2001 Dec 29;356(1416):1895-7. doi: 10.1098/rstb.2001.1002. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11779389 Free PMC article. Review.
Cited by
-
Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors.Antimicrob Agents Chemother. 2005 Nov;49(11):4515-20. doi: 10.1128/AAC.49.11.4515-4520.2005. Antimicrob Agents Chemother. 2005. PMID: 16251290 Free PMC article.
-
Phenotypic drug susceptibility assay for influenza virus neuraminidase inhibitors.Clin Diagn Lab Immunol. 2004 Jan;11(1):21-8. doi: 10.1128/cdli.11.1.21-28.2004. Clin Diagn Lab Immunol. 2004. PMID: 14715540 Free PMC article.
-
Management of influenza virus infections with neuraminidase inhibitors: detection, incidence, and implications of drug resistance.Treat Respir Med. 2005;4(2):107-16. doi: 10.2165/00151829-200504020-00004. Treat Respir Med. 2005. PMID: 15813662 Free PMC article. Review.
-
Evaluation of PD 404,182 as an anti-HIV and anti-herpes simplex virus microbicide.Antimicrob Agents Chemother. 2014;58(2):687-97. doi: 10.1128/AAC.02000-13. Epub 2013 Nov 11. Antimicrob Agents Chemother. 2014. PMID: 24217696 Free PMC article.
-
Antiviral combinations for severe influenza.Lancet Infect Dis. 2014 Dec;14(12):1259-70. doi: 10.1016/S1473-3099(14)70821-7. Epub 2014 Sep 8. Lancet Infect Dis. 2014. PMID: 25213733 Free PMC article. Review.
References
-
- Babu, Y. S., P. Chand, S. Bantia, P. Kotian, A. Dehghani, Y. El-Kattan, T-H. Lin, T. L. Hutchison, A. J. Elliott, C. D. Parker, S. L. Ananth, L. L. Horn, G. W. Laver, and J. A. Montgomery. 2000. BCX-1812: discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43:3482-3486. - PubMed
-
- Bantia, S., C. D. Parker, S. L. Ananth, L. L. Horn, K. Andries, P. Chand, P. L. Kotian, A. Dehghani, Y. El-Kattan, T. Lin, T. L. Hutchison, J. A. Montgomery, D. L. Kellog, and Y. S. Babu. 2001. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162-1167 - PMC - PubMed
-
- Barnett, J. M., A. Cadman, F. M. Burrell, S. H. Madar, A. P. Lewis, M. Tisdale, and R. Bethell. 1999. In vitro selection and characterization of influenza B/Beijing/1/87 isolates with altered susceptibility to zanamivir. Virology 265:286-295. - PubMed
-
- Blick, T. J., T. Tiong, A. Sahasrabudhe, J. N. Varghese, P. M. Colman, G. J. Hart, R. C. Bethell, and J. L. McKimm-Breschkin. 1995. Generation and characterization of an influenza virus neuraminidase variant with decreased sensitivity for the neuraminidase-specific inhibitor 4-guanidino-neu5Ac2en. Virology 214:475-484. - PubMed
-
- Blick, T. J., A. Sahasrabudhe, M. McDonald, I. J. Owens, P. J. Morley, R. J. Fenton, and J. L. McKimm-Breschkin. 1998. The interaction of neuraminidase and hemagglutinin mutations in influenza virus in resistance to 4-guanidino-Neu5Ac2en. Virology 246:95-103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

