Transduction of interphase cells by avian sarcoma virus
- PMID: 11991971
- PMCID: PMC137034
- DOI: 10.1128/jvi.76.11.5422-5434.2002
Transduction of interphase cells by avian sarcoma virus
Abstract
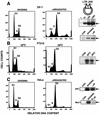
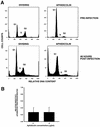
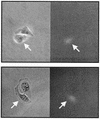
It has been generally believed that oncoretroviruses are dependent on mitosis for efficient nuclear entry of viral DNA. We previously identified a nuclear localization signal in the integrase protein of an oncoretrovirus, avian sarcoma virus (ASV), suggesting an active import mechanism for the integrase-DNA complex (G. Kukolj, R. A. Katz, and A. M. Skalka, Gene 223:157-163, 1998). Here, we have evaluated the requirement for mitosis in nuclear import and integration of ASV DNA. Using a modified ASV encoding a murine leukemia virus amphotropic env gene and a green fluorescent protein (GFP) reporter gene, DNA nuclear import was measured in cell cycle-arrested avian (DF-1) as well as human (HeLa) and mouse cells. The results showed efficient accumulation of nuclear forms of ASV DNA in gamma-irradiation-arrested cells. Efficient transduction of a GFP reporter gene was also observed after infection of cells that were arrested with gamma-irradiation, mitomycin C, nocodazole, or aphidicolin, confirming that nuclear import and integration of ASV DNA can occur in the absence of mitosis. By monitoring GFP expression in individual cells, we also obtained evidence for nuclear import of viral DNA during interphase in cycling cells. Lastly, we observed that ASV can transduce postmitotic mouse neurons. These results support an active nuclear import mechanism for the oncoretrovirus ASV and suggest that this mechanism can operate in both nondividing and dividing cells.
Figures
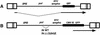




References
-
- Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

