Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion
- PMID: 11992000
- PMCID: PMC137054
- DOI: 10.1128/jvi.76.11.5720-5728.2002
Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion
Abstract
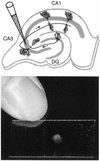
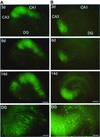
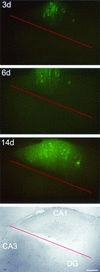
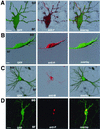
Measles virus (MV) can infect the central nervous system and, in rare cases, causes subacute sclerosing panencephalitis, characterized by a progressive degeneration of neurons. The route of MV transmission in neurons was investigated in cultured rat hippocampal slices by using MV expressing green fluorescent protein. MV infected hippocampal neurons and spread unidirectionally, in a retrograde manner, from CA1 to CA3 pyramidal cells and from there to the dentate gyrus. Spreading of infection depended on cell-to-cell contact and occurred without any detectable release of infectious particles. The role of the viral proteins in the retrograde MV transmission was determined by investigating their sorting in infected pyramidal cells. MV glycoproteins, the fusion protein (F) and hemagglutinin (H), the matrix protein (M), and the phosphoprotein (P), which is part of the viral ribonucleoprotein complex, were all sorted to the dendrites. While M, P, and H proteins remained more intracellular, the F protein localized to prominent, spine-type domains at the surface of infected cells. The detected localization of MV proteins suggests that local microfusion events may be mediated by the F protein at sites of synaptic contacts and is consistent with a mechanism of retrograde transmission of MV infection.
Figures
References
-
- Baczko, K., J. Lampe, U. G. Liebert, U. Brinckmann, V. ter Meulen, I. Pardowitz, H. Budka, S. L. Cosby, S. Isserte, and B. K. Rima. 1993. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology 197:188-195. - PubMed
-
- Billeter, M. A., and R. Cattaneo. 1991. Molecular biology of defective measles viruses persisting in the human central nervous system, p. 323-345. In D. Kingsbury (ed.), The paromyxoviruses. Plenum Press, New York, N.Y.
-
- Blau, D. M., and R. W. Compans. 1995. Entry and release of measles virus are polarized in epithelial cells. Virology 210:91-99. - PubMed
-
- Burack, M. A., M. A. Silverman, and G. Banker. 2000. The role of selective transport in neuronal protein sorting. Neuron 26:465-472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

