Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats
- PMID: 11992016
- PMCID: PMC155932
- DOI: 10.1101/lm.46102
Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats
Abstract
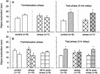
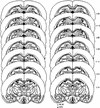
Exposures to uncontrollable stress have been shown to alter ensuing synaptic plasticity in the hippocampus and interfere with hippocampal-dependent spatial memory in rats. The present study examined whether stress, which impairs hippocampal long-term potentiation (LTP), also affects (nonspatial) hippocampal-dependent object-recognition memory, as tested on the visual paired comparison task (VPC) in rats. After undergoing an inescapable restraint-tailshock stress experience, rats exhibited markedly impaired recognition memory at the 3-h (long) familiarization-to-test phase delay but not at the 5-min (short) delay. In contrast, unstressed control animals showed robust recognition memory (i.e., they exhibited reliable preferences for novel over familiar objects) at both short- and long-delay periods. The impairing effect of stress on long-delay recognition memory was transient because 48 h after undergoing stress experience, animals performed normally at the long delay. Similar to stress, microinfusions of DL-2-amino-5-phosphonovaleric acid (APV), a competitive N-methyl-D-aspartate receptor (NMDAR) antagonist that blocks LTP, into the dorsal hippocampus selectively impaired object-recognition memory at the long-delay period. Together, these results suggest that stress and intrahippocampal administration of APV affect recognition memory by influencing synaptic plasticity in the hippocampus.
Figures


References
-
- Anderson BJ, Steinmetz JE. Cerebellar and brainstem circuits involved in classical eyeblink conditioning. Rev Neurosci. 1994;5:251–273. - PubMed
-
- Bachevalier J, Brickson M, Hagger C, Mishkin M. Age and sex differences in the effects of selective temporal lobe lesion on the formation of visual discrimination habits in rhesus monkeys (Macaca mulatta) Behav Neurosci. 1990;104:885–899. - PubMed
-
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. - PubMed
-
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Brain Res. 1999;830:56–71. - PubMed
-
- Beylin AV, Shors TJ. Stress enhances excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav Neurosci. 1998;112:1327–1338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
