Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis
- PMID: 11994412
- PMCID: PMC150963
- DOI: 10.1172/JCI14698
Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis
Abstract
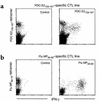
While the pathologic mechanisms responsible for organ-specific tissue damage in primary biliary cirrhosis (PBC) remain an enigma, it has been suggested that the pathology is mediated by autoreactive T cells infiltrating the intrahepatic bile ducts. Previously, we have documented that there is 100-fold enrichment in the frequency of CD4(+) autoreactive T cells in the liver that are specific for peptides encoded by the E2 components of the pyruvate dehydrogenase complexes (PDC-E2). We have also recently characterized the first MHC class I-restricted epitope for PDC-E2, namely amino acid 159-167, a region very similar to the epitope recognized by MHC class II-restricted CD4(+) cells and by autoantibodies. The effector functions of these PDC-E2(159-167)-specific CD8(+) cytotoxic T lymphocytes (CTLs) are not well understood. We have taken advantage of tetramer technology and report herein that there is tenfold increase in the frequency of PDC-E2(159-167)-specific CTLs in the liver as compared with the blood in PBC. In addition, the precursor frequency of the CTLs in blood was significantly higher in early-stage PBC. Of interest was the fact that, upon stimulation with the peptide, the response of PDC-E2(159-167) tetramer-positive cells is heterogeneous with respect to IFN-gamma synthesis. These data, we believe for the first time, document the enrichment of autoantigen-specific CD8(+) T cells in the PBC liver, suggesting that CD8(+) T cells play a significant role in the immunopathogenesis of PBC.
Figures


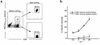

References
-
- Coppel RL, Gershwin ME. Primary biliary cirrhosis: the molecule and the mimic. Immunol Rev. 1995; 144:17–49. - PubMed
-
- Van de Water J, et al. The role of T cells in primary biliary cirrhosis. Semin Liver Dis. 1997; 17:105–113. - PubMed
-
- Colucci G, Schaffner F, Paronetto F. In situ characterization of the cell-surface antigens of the mononuclear cell infiltrate and bile duct epithelium in primary biliary cirrhosis. Clin Immunol Immunopathol. 1986; 41:35–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

