Hematopoietic stem cells are uniquely selective in their migratory response to chemokines
- PMID: 11994419
- PMCID: PMC2193709
- DOI: 10.1084/jem.20011284
Hematopoietic stem cells are uniquely selective in their migratory response to chemokines
Abstract
Although hematopoietic stem cell (HSC) migration into and out of sites of active hematopoiesis is poorly understood, it is a critical process that underlies modern clinical stem cell transplantation and may be important for normal hematopoietic homeostasis. Given the established roles of chemotactic cytokine (chemokine)-directed migration of other leukocyte subsets, the migration of murine HSC to a large panel of CC and CXC chemokines was investigated. HSC migrated only in response to stromal derived factor-1alpha, the ligand for the CXC chemokine receptor 4 (CXCR4). CXCR4 expression by HSC was confirmed by reverse transcription polymerase chain reaction analysis. Surprisingly, HSC also expressed mRNA for CCR3 and CCR9, although they failed to migrate to the ligands for these receptors. The sharply restricted chemotactic responsiveness of HSC is unique among leukocytes and may be necessary for the specific homing of circulating HSC to bone marrow, as well as for the maintenance of HSC in hematopoietic microenvironments.
Figures






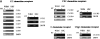
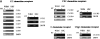
References
-
- Morrison, S.J., N. Uchida, and I.L. Weissman. 1995. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11:35–71. - PubMed
-
- Ley, K., editor. 2001. Physiology of Inflammation. Oxford University Press, New York. 546 pp.
-
- Goodman, J.W., and G.S. Hodgson. 1962. Evidence for stem cells in the peripheral blood of mice. Blood. 19:702–714. - PubMed
-
- Wright, D.E., A.J. Wagers, A.P. Gulati, F.L. Johnson, and I.L. Weissman. 2001. Physiological migration of hematopoietic stem and progenitor cells. Science. 294:1933–1936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- 5T32 AI-07290/AI/NIAID NIH HHS/United States
- R37 GM037734/GM/NIGMS NIH HHS/United States
- R37 AI047822/AI/NIAID NIH HHS/United States
- T32 CA009151/CA/NCI NIH HHS/United States
- GM37734/GM/NIGMS NIH HHS/United States
- AI47822/AI/NIAID NIH HHS/United States
- 5R01 HL58770/HL/NHLBI NIH HHS/United States
- R01 GM037734/GM/NIGMS NIH HHS/United States
- R21 AI047822/AI/NIAID NIH HHS/United States
- R01 AI047822/AI/NIAID NIH HHS/United States
- R01 HL058770/HL/NHLBI NIH HHS/United States
- 5T32CA09151/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

