Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates
- PMID: 11994420
- PMCID: PMC2193703
- DOI: 10.1084/jem.20011547
Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates
Abstract
Salmonella typhimurium causes an invasive disease in mice that has similarities to human typhoid. A type III protein secretion system encoded by Salmonella pathogenicity island 2 (SPI2) is essential for virulence in mice, as well as survival and multiplication within macrophages. Reactive nitrogen intermediates (RNI) synthesized by inducible nitric oxide synthase (iNOS) are involved in the control of intracellular pathogens, including S. typhimurium. We studied the effect of Salmonella infection on iNOS activity in macrophages. Immunofluorescence microscopy demonstrated efficient colocalization of iNOS with bacteria deficient in SPI2 but not wild-type Salmonella, and suggests that the SPI2 system interferes with the localization of iNOS and Salmonella. Furthermore, localization of nitrotyrosine residues in the proximity was observed for SPI2 mutant strains but not wild-type Salmonella, indicating that peroxynitrite, a potent antimicrobial compound, is excluded from Salmonella-containing vacuoles by action of SPI2. Altered colocalization of iNOS with intracellular Salmonella required the function of the SPI2-encoded type III secretion system, but not of an individual "Salmonella translocated effector." Inhibition of iNOS increased intracellular proliferation of SPI2 mutant bacteria and, to a lesser extent, of wild-type Salmonella. The defect in systemic infection of a SPI2 mutant strain was partially restored in iNOS(-/-) mice. In addition to various strategies to detoxify RNI or repair damage due to RNI, avoidance of colocalization with RNI is important in adaptation of a pathogen to an intracellular life style.
Figures

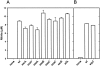

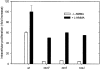




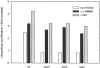
Comment in
-
Salmonella selectively stops traffic.Trends Microbiol. 2002 Sep;10(9):391-2. doi: 10.1016/s0966-842x(02)02423-x. Trends Microbiol. 2002. PMID: 12217496
References
-
- Shiloh, M.U., J.D. MacMicking, S. Nicholson, J.E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 10:29–38. - PubMed
-
- Galan, J.E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53–86. - PubMed
-
- Wallis, T.S., and E.E. Galyov. 2000. Molecular basis of Salmonella-induced enteritidis. Mol. Microbiol. 36:997–1005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

