The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound
- PMID: 11994426
- PMCID: PMC2193707
- DOI: 10.1084/jem.20011783
The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound
Abstract
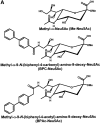
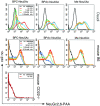
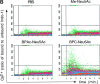
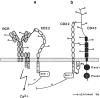
CD22 is a B cell-specific transmembrane protein of the Siglec family. It binds specifically to alpha2,6-linked sialic acid (Sia) residues, which are also present on glycoproteins on the B cell surface. CD22 acts as a negative regulator in B cell receptor-mediated signaling by recruitment of Src homology 2 domain-containing tyrosine phosphatase (SHP)-1 to its intracellular tail. To analyze how ligand-binding of CD22 influences its intracellular signaling domain, we designed synthetic sialosides as inhibitors for the lectin domain of CD22. One of these compounds inhibited binding of human CD22-Fc to target cells over 200-fold better than Sia and was highly selective for human CD22. When Daudi cells or primary B cells were stimulated with anti-immunoglobulin (Ig)M in presence of this sialoside inhibitor, a higher Ca(2+) response was observed, similar to CD22-deficient B cells. Accordingly, a lower tyrosine-phosphorylation of CD22 and SHP-1 recruitment was demonstrated in presence of the sialoside. Thus, by interfering with ligand binding of CD22 on the B cell surface, we have shown for the first time that the lectin domain of CD22 has a direct, positive influence on its intracellular inhibitory domain. Also, we have developed a novel low molecular weight compound which can enhance the response of human B cells.
Figures




References
-
- Nitschke, L., R. Carsetti, B. Ocker, G. Köhler, and M.C. Lamers. 1997. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 7:133–143. - PubMed
-
- O'Keefe, T.L., G.T. Williams, S.L. Davies, and M.S. Neuberger. 1996. Hyperresponsive B cells in CD22-deficient mice. Science. 274:798–801. - PubMed
-
- Otipoby, K.L., K.B. Andersson, K.E. Draves, S.J. Klaus, A.G. Farr, J.D. Kerner, R.M. Perlmutter, C.L. Law, and E.A. Clark. 1996. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 384:634–637. - PubMed
-
- Sato, S., A.S. Miller, M. Inaoki, C.B. Bock, P.J. Jansen, M.L. Tang, and T.F. Tedder. 1996. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 5:551–562. - PubMed
-
- Doody, G.M., L.B. Justement, C.C. Delibrias, R.J. Matthews, J. Lin, M.L. Thomas, and D.T. Fearon. 1995. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 269:242–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

