Mayolenes: labile defensive lipids from the glandular hairs of a caterpillar (Pieris rapae)
- PMID: 11997469
- PMCID: PMC124487
- DOI: 10.1073/pnas.102165699
Mayolenes: labile defensive lipids from the glandular hairs of a caterpillar (Pieris rapae)
Abstract
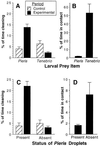
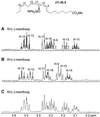
Larvae of the European cabbage butterfly, Pieris rapae (Pieridae), are beset with glandular hairs, bearing droplets of a clear oily secretion at their tip. The fluid consists primarily of a series of chemically labile, unsaturated lipids, the mayolenes, which are derived from 11-hydroxylinolenic acid. In bioassays with the ant Crematogaster lineolata, the secretion was shown to be potently deterrent, indicating that the fluid plays a defensive role in nature.
Figures





References
-
- Opler P A, Krizek G O. Butterflies East of the Great Plains. Baltimore: Johns Hopkins Univ. Press; 1984.
-
- Newman E. An Illustrated Natural History of British Butterflies. London: W. Tweedie; 1871.
-
- Griesinger C, Sorensen O W, Ernst R R. J Magn Reson. 1987;75:474–492.
-
- Weibel, D. B., Shevy, L. E., Schroeder, F. C. & Meinwald, J. (2002) J. Org. Chem., in press. - PubMed
-
- Dale J A, Dull D L, Mosher H S. J Org Chem. 1969;34:2543–2549.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

