Induced expression and association of the Mona/Gads adapter and Gab3 scaffolding protein during monocyte/macrophage differentiation
- PMID: 11997510
- PMCID: PMC133813
- DOI: 10.1128/MCB.22.11.3744-3756.2002
Induced expression and association of the Mona/Gads adapter and Gab3 scaffolding protein during monocyte/macrophage differentiation
Abstract
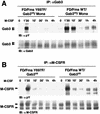
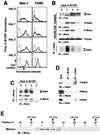
Mona/Gads is a Grb2-related, Src homology 3 (SH3) and SH2 domain-containing adapter protein whose expression is restricted to cells of hematopoietic lineage (i.e., monocytes and T lymphocytes). During monocyte/macrophage differentiation, Mona is induced and interacts with the macrophage colony-stimulating factor receptor, M-CSFR (also called Fms), suggesting that Mona could be involved in developmental signaling downstream of the M-CSFR by recruiting additional signaling proteins to the activated receptor. Our present results identify Mona as a specific partner protein for the DOS/Gab family member Gab3 in monocytic/macrophage development. Mona does not interact with Gab2; however, Gab3 also forms a complex with the Mona-related adapter Grb2. Glutathione S-transferase pull-down experiments demonstrate that the Mona and Gab3 interaction utilizes the carboxy-terminal SH3 domain of Mona and the atypical proline-rich domain of Gab3. Mona is known to interact with the phosphorylated Y697 site of the M-CSFR. The M-CSFR mutation Y697F exhibited qualitative and quantitative abnormalities in receptor and Gab3 tyrosine phosphorylation, and Mona induction was greatly reduced. The Y807F M-CSFR mutation is defective in differentiation signaling, but not growth signaling, and also fails to induce Mona protein expression. During M-CSF-stimulated macrophage differentiation of mouse bone marrow cells, Mona and Gab3 expression is coinduced, these proteins interact, and Mona engages in multimolecular complexes. These data suggest that association of Mona and Gab3 plays a specific role in mediating the M-CSFR differentiation signal.
Figures






Similar articles
-
Mona, a novel hematopoietic-specific adaptor interacting with the macrophage colony-stimulating factor receptor, is implicated in monocyte/macrophage development.EMBO J. 1998 Dec 15;17(24):7273-81. doi: 10.1093/emboj/17.24.7273. EMBO J. 1998. PMID: 9857184 Free PMC article.
-
Gab3, a new DOS/Gab family member, facilitates macrophage differentiation.Mol Cell Biol. 2002 Jan;22(1):231-44. doi: 10.1128/MCB.22.1.231-244.2002. Mol Cell Biol. 2002. PMID: 11739737 Free PMC article.
-
Expression of Mona (monocytic adapter) in myeloid progenitor cells results in increased and prolonged MAP kinase activation upon macrophage colony-stimulating factor stimulation.FEBS Lett. 2000 Sep 1;480(2-3):113-7. doi: 10.1016/s0014-5793(00)01906-2. FEBS Lett. 2000. PMID: 11034310
-
Growth and differentiation signals regulated by the M-CSF receptor.Mol Reprod Dev. 1997 Jan;46(1):96-103. doi: 10.1002/(SICI)1098-2795(199701)46:1<96::AID-MRD15>3.0.CO;2-1. Mol Reprod Dev. 1997. PMID: 8981370 Review.
-
CSF-1 signal transduction.J Leukoc Biol. 1997 Aug;62(2):145-55. doi: 10.1002/jlb.62.2.145. J Leukoc Biol. 1997. PMID: 9261328 Review.
Cited by
-
Structural basis for SH3 domain-mediated high-affinity binding between Mona/Gads and SLP-76.EMBO J. 2003 Jun 2;22(11):2571-82. doi: 10.1093/emboj/cdg258. EMBO J. 2003. PMID: 12773374 Free PMC article.
-
SeXY chromosomes and the immune system: reflections after a comparative study.Biol Sex Differ. 2020 Jan 14;11(1):3. doi: 10.1186/s13293-019-0278-y. Biol Sex Differ. 2020. PMID: 31937374 Free PMC article.
-
Identification of differential protein expression associated with development of unstable human carotid plaques.Am J Pathol. 2006 Mar;168(3):1004-21. doi: 10.2353/ajpath.2006.050471. Am J Pathol. 2006. PMID: 16507914 Free PMC article.
-
CSF-1 receptor signaling in myeloid cells.Cold Spring Harb Perspect Biol. 2014 Jun 2;6(6):a021857. doi: 10.1101/cshperspect.a021857. Cold Spring Harb Perspect Biol. 2014. PMID: 24890514 Free PMC article. Review.
-
Itk Promotes the Integration of TCR and CD28 Costimulation through Its Direct Substrates SLP-76 and Gads.J Immunol. 2021 May 15;206(10):2322-2337. doi: 10.4049/jimmunol.2001053. Epub 2021 Apr 30. J Immunol. 2021. PMID: 33931484 Free PMC article.
References
-
- Asakura, E., T. Yamauchi, A. Umemura, T. Hanamura, and T. Tanabe. 1997. Intravenously administered macrophage colony-stimulating factor (M-CSF) specifically acts on the spleen, resulting in the increasing and activating spleen macrophages for cytokine production in mice. Immunopharmacology 37:7-14. - PubMed
-
- Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. - PubMed
-
- Bardelli, A., P. Longati, D. Gramaglia, M. C. Stella, and P. M. Comoglio. 1997. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene 15:3103-3111. - PubMed
-
- Berry, D. M., S. J. Benn, A. M. Cheng, and C. J. McGlade. 2001. Caspase-dependent cleavage of the hematopoietic specific adapter Gads alters signaling from the T cell receptor. Oncogene 20:1203-1211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
