The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas
- PMID: 11997520
- PMCID: PMC133833
- DOI: 10.1128/MCB.22.11.3864-3874.2002
The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas
Abstract
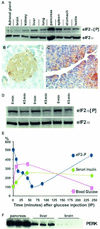
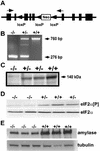
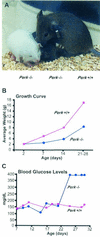
Phosphorylation of eukaryotic initiation factor 2 alpha (eIF-2 alpha) is typically associated with stress responses and causes a reduction in protein synthesis. However, we found high phosphorylated eIF-2 alpha (eIF-2 alpha[P]) levels in nonstressed pancreata of mice. Administration of glucose stimulated a rapid dephosphorylation of eIF-2 alpha. Among the four eIF-2 alpha kinases present in mammals, PERK is most highly expressed in the pancreas, suggesting that it may be responsible for the high eIF-2 alpha[P] levels found therein. We describe a Perk knockout mutation in mice. Pancreata of Perk(-/-) mice are morphologically and functionally normal at birth, but the islets of Langerhans progressively degenerate, resulting in loss of insulin-secreting beta cells and development of diabetes mellitus, followed later by loss of glucagon-secreting alpha cells. The exocrine pancreas exhibits a reduction in the synthesis of several major digestive enzymes and succumbs to massive apoptosis after the fourth postnatal week. Perk(-/-) mice also exhibit skeletal dysplasias at birth and postnatal growth retardation. Skeletal defects include deficient mineralization, osteoporosis, and abnormal compact bone development. The skeletal and pancreatic defects are associated with defects in the rough endoplasmic reticulum of the major secretory cells that comprise the skeletal system and pancreas. The skeletal, pancreatic, and growth defects are similar to those seen in human Wolcott-Rallison syndrome.
Figures




References
-
- Abraham, N., D. F. Stojdl, P. I. Duncan, N. Methot, T. Ishii, M. Dube, B. C. Vanderhyden, H. L. Atkins, D. A. Gray, M. W. McBurney, A. E. Koromilas, E. G. Brown, N. Sonenberg, and J. C. Bell. 1999. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 274:5953-5962. - PubMed
-
- Berlanga, J. J., J. Santoyo, and C. De Haro. 1999. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2α kinase. Eur. J. Biochem. 265:754-762. - PubMed
-
- Bertolotti, A., Y. Zhang, L. M. Hendershot, H. P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326-332. - PubMed
-
- Casini, A., A. Galli, P. Pignalosa, L. Frulloni, C. Grappone, S. Milani, P. Pederzoli, G. Cavallini, and C. Surrenti. 2000. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J. Pathol. 192:81-89. - PubMed
-
- Chen, J. J., and I. M. London. 1995. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem. Sci. 20:105-108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
