Suppression of diabetic retinopathy with angiopoietin-1
- PMID: 12000720
- PMCID: PMC1850865
- DOI: 10.1016/S0002-9440(10)61115-7
Suppression of diabetic retinopathy with angiopoietin-1
Abstract
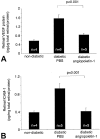
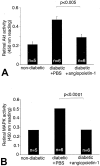
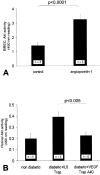
Diabetic retinopathy remains a leading cause of irreversible blindness. A critical early pathology in the disease is the adhesion of leukocytes to the retinal vasculature, a process that occurs, in part, via intercellular adhesion molecule-1. Once leukocyte adhesion occurs, endothelial cell injury ensues, as does blood-retinal barrier breakdown. Here we show that angiopoietin-1 can prevent and reverse these diabetic retinal vascular changes in both new and established diabetes. Angiopoietin-1, when given intravitreally to newly diabetic rats, normalized retinal vascular endothelial growth factor (VEGF) and intercellular adhesion molecule-1 mRNA and protein levels, leading to reductions in leukocyte adhesion, endothelial cell injury, and blood-retinal barrier breakdown. When an adenovirus coding for angiopoietin-1 was given systemically to mice with established diabetes, it similarly inhibited leukocyte adhesion and endothelial cell injury and blood-retinal barrier breakdown. These changes coincided with reductions in retinal eNOS, nitric oxide, Akt (protein kinase B), and MAP kinase activity, known mediators of VEGF bioactivity and leukocyte adhesion. When endogenous VEGF bioactivity was inhibited with a soluble Flt-1/Fc chimera, retinal Akt kinase activity was significantly reduced in vivo. Taken together, these data document new vascular and anti-inflammatory bioactivities for angiopoietin-1 and identify it as the first naturally occurring protein that directly protects the retinal vasculature in diabetes.
Figures




Comment in
-
Sugar creates a sticky business: round up the usual suspects.Am J Pathol. 2002 May;160(5):1547-50. doi: 10.1016/S0002-9440(10)61099-1. Am J Pathol. 2002. PMID: 12000704 Free PMC article. No abstract available.
References
-
- Miyamoto K, Khosrof S, Bursell S-E, Rohan R, Murata T, Clermont A, Aiello LP, Ogura Y, Adamis AP: Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA 1999, 96:10836-10841 - PMC - PubMed
-
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996, 87:1161-1169 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

