Altered Notch ligand expression in human liver disease: further evidence for a role of the Notch signaling pathway in hepatic neovascularization and biliary ductular defects
- PMID: 12000721
- PMCID: PMC1850864
- DOI: 10.1016/S0002-9440(10)61116-9
Altered Notch ligand expression in human liver disease: further evidence for a role of the Notch signaling pathway in hepatic neovascularization and biliary ductular defects
Abstract
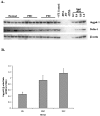
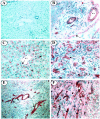
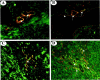
The Jagged and Delta family of transmembrane proteins are ligands for Notch receptors, which control the proliferation and/or differentiation of many cell lineages. Expression and localization of these ligands in the adult human liver has not been fully elucidated, nor whether dysregulation of these proteins contributes to liver disease processes. We have examined expression of the five known Notch ligands in human liver. Expression of Jagged-1 and Delta-4 mRNA was seen in normal and diseased liver tissue, whereas Jagged-2, Delta-1, and Delta-3 mRNA was undetectable. In primary liver cell isolates, Jagged-1 expression was found in all cell types, whereas Delta-4 was present in biliary epithelial and liver endothelial cells, but absent in hepatocytes. Interestingly, Jagged-1 mRNA expression was significantly up-regulated in diseased liver tissue. By immunohistochemistry, Jagged-1 expression was present on most structures in normal tissue. However in disease, strikingly strong Jagged-1 immunoreactivity was observed on many small neovessels and bile ductules. The expression of downstream modulators and effectors of Notch signaling was also detectable in purified cell isolates. This, together with aberrant Jagged-1 expression suggests that the Notch signaling pathway may play a role in the neovascularization and biliary defects observed in the liver during the development of cirrhosis.
Figures


References
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ: Notch signaling: cell fate control and signal integration in development. Science 1999, 284:770-776 - PubMed
-
- Mumm JS, Kopan R: Notch signaling: from the outside in. Dev Biol 2000, 228:151-165 - PubMed
-
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J: TAN-1, the human homologue of the Drosophila gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991, 66:649-661 - PubMed
-
- Stifani S, Blaumueller CM, Redhead NJ, Hill RE, Artavanis-Tsakonas S: Human homologs of a Drosophila enhancer of split gene product define a novel family of nuclear proteins. Nat Genet 1992, 2:119-127 - PubMed
-
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis E-A, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E: Notch3 mutations in CADASIL, an hereditary adult onset condition causing stroke and dementia. Nature 1996, 383:707-710 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

