Carp, a cardiac ankyrin-repeated protein, and its new homologue, Arpp, are differentially expressed in heart, skeletal muscle, and rhabdomyosarcomas
- PMID: 12000728
- PMCID: PMC1850855
- DOI: 10.1016/S0002-9440(10)61123-6
Carp, a cardiac ankyrin-repeated protein, and its new homologue, Arpp, are differentially expressed in heart, skeletal muscle, and rhabdomyosarcomas
Abstract
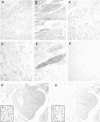
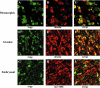
Arpp, a protein containing an ankyrin repeat domain, PEST sequence, and proline-rich region, is a novel ankyrin-repeated protein highly homologous to Carp, which is proposed to be the putative genetic marker for cardiac hypertrophy. In this study, we comparatively analyzed expression of Arpp and Carp protein in skeletal and cardiac muscles and rhabdomyosarcomas (RMSs). In adult skeletal muscle, Arpp was preferentially expressed in the nucleus and cytoplasm of type I fibers, whereas Carp was barely detectable in skeletal muscle. On the other hand, in adult cardiac muscle, interestingly, Arpp was expressed in ventricles mostly, whereas Carp was expressed throughout the atrium and ventricle. Furthermore, although Carp was identified in fetal heart at 11 developmental weeks, Arpp was very low or undetectable in these fetal hearts. These results suggest that Arpp and Carp are differentially expressed and function in both skeletal and cardiac muscle of fetus and adult. We found that Arpp expression was induced during the differentiation of C2C12 cells in vitro, suggesting that Arpp-expression may be associated with the differentiation stage during myogenesis. Both Arpp and Carp were found to be expressed in all of the RMS cases studied. Because the expression patterns of Arpp in RMS were different from those of muscle actin or desmin, Arpp may be detectable in RMS cases that do not express other existing RMS markers.
Figures





References
-
- Moriyama M, Tsukamoto Y, Fujiwara M, Kondo G, Nakada C, Baba T, Ishiguro N, Miyazaki A, Nakamura K, Hori N, Sato K, Shomori K, Takeuchi K, Satoh H, Mori S, Ito H: Identification of a novel human ankyrin-repeated protein homologous to CARP. Biochem Biophys Res Comm 2001, 285:715-723 - PubMed
-
- Kemp TJ, Sadusky TJ, Saltisi F, Carey N, Moss J, Yang SY, Sassoon DA, Goldspink G, Coulton GR: Identification of Ankrd2, a novel skeletal muscle gene coding for a stretch-responsive ankyrin-repeat protein. Genomics 2000, 66:229-241 - PubMed
-
- Zou Y, Evans S, Chen J, Kuo H-C, Harvey RP, Chien KR: CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development 1997, 124:793-804 - PubMed
-
- Kuo H, Chen J, Ruiz-Lozano P, Zou Y, Nemer M, Chien KR: Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development 1999, 126:4223-4234 - PubMed
-
- Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L: A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem 1997, 272:22800-22808 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

