Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis
- PMID: 12000730
- PMCID: PMC1850856
- DOI: 10.1016/s0002-9440(10)61125-x
Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis
Expression of concern in
-
Note of Concern.Am J Pathol. 2018 Jan;188(1):265. doi: 10.1016/j.ajpath.2017.10.002. Am J Pathol. 2018. PMID: 29249254 Free PMC article. No abstract available.
Abstract
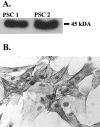
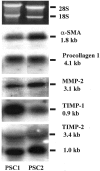
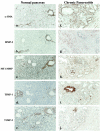
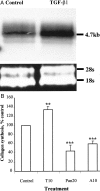
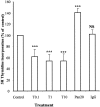
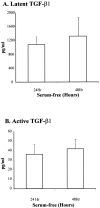
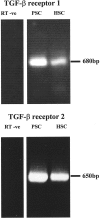
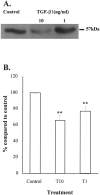
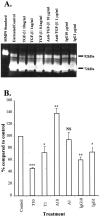
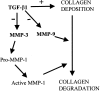
Pancreatic stellate cells mediate fibrosis in chronic pancreatitis. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs)-1 and -2 are crucial modulators of fibrosis. Transforming growth factor-beta (TGF-beta) is a key regulator of extracellular matrix production and myofibroblast proliferation. We have examined MMP and TIMP synthesis by transformed cultured pancreatic stellate cells and their regulation by TGF-beta 1. By Northern analysis they expressed mRNAs for procollagen 1, TIMP-1, TIMP-2, and MMP-2. Expression of membrane type-1 MMP was confirmed by Western blotting. By immunohistochemistry these enzymes localized to fibrotic areas in human chronic pancreatitis. Active TGF-beta 1 constitutes 2 to 5% of total TGF-beta 1 secreted by pancreatic stellate cells; they express TGF-beta receptors I and II. Exogenous TGF-beta 1 (10 ng/ml) significantly increased procollagen-1 mRNA by 69% and collagen protein synthesis by 34%. Similarly TGF-beta 1 at 0.1, 1, and 10 ng/ml significantly reduced cellular proliferation rate by 37%, 44%, and 44%, respectively, whereas pan-TGF-beta-neutralizing antibody increased proliferation by 40%. TGF-beta1 (10 ng/ml) down-regulated MMP-9 by 54% and MMP-3 by 34% whereas TGF-beta 1-neutralizing antibody increased MMP-9 expression by 39%. Pancreatic stellate cells express both mediators of matrix remodeling and the regulatory cytokine TGF-beta 1 that, by autocrine inhibition of MMP-3 and MMP-9, may enhance fibrogenesis by reducing collagen degradation.
Figures

References
-
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G: Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115:421-432 - PubMed
-
- Friedman SL: Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 1993, 328:1828-1835 - PubMed
-
- Arthur MJ: Collagenases and liver fibrosis. J Hepatol 1995, 22:43-48 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

