Targets of AtWRKY6 regulation during plant senescence and pathogen defense
- PMID: 12000796
- PMCID: PMC186251
- DOI: 10.1101/gad.222702
Targets of AtWRKY6 regulation during plant senescence and pathogen defense
Abstract
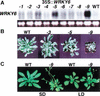
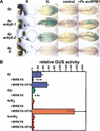
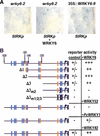
In Arabidopsis, WRKY factors comprise a large gene family of plant-specific transcriptional regulators controlling several types of plant stress responses. To understand the regulatory role of WRKY proteins during such processes, we identified targets of the senescence- and defense-associated WRKY6 factor. WRKY6 was found to suppress its own promoter activity as well as that of a closely related WRKY family member, indicating negative autoregulation. On the other hand, WRKY6 positively influenced the senescence- and pathogen defense-associated PR1 promoter activity, most likely involving NPR1 function. One novel identified target gene, SIRK, encodes a receptor-like protein kinase, whose developmental expression is strongly induced specifically during leaf senescence. The transcriptional activation of SIRK is dependent on WRKY6 function. Senescing leaves of wrky6 knockout mutants showed a drastic reduction, and green leaves of WRKY6 overexpression lines showed clearly elevated SIRK transcript levels. Furthermore, the SIRK gene promoter was specifically activated by WRKY6 in vivo, functioning very likely through direct W-box interactions.
Figures



References
-
- Baumann E, Lewald J, Saedler H, Schulz B, Wisman E. Successful PCR based reverse genetic screens using an En-1 mutagenised Arabidopsis thaliana population generated via single-seed descent. Theor Appl Genet. 1998;97:729–734.
-
- Bouche N, Bouchez D. Arabidopsis gene knockout: Phenotypes wanted. Curr Opin Plant Biol. 2001;4:111–117. - PubMed
-
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. - PubMed
-
- Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
