Nitric oxide metabolism in Neisseria meningitidis
- PMID: 12003939
- PMCID: PMC135047
- DOI: 10.1128/JB.184.11.2987-2993.2002
Nitric oxide metabolism in Neisseria meningitidis
Abstract
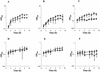
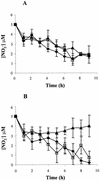
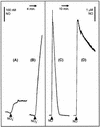
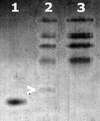
Neisseria meningitidis, the causative agent of meningococcal disease in humans, is likely to be exposed to nitrosative stress during natural colonization and disease. The genome of N. meningitidis includes the genes aniA and norB, predicted to encode nitrite reductase and nitric oxide (NO) reductase, respectively. These gene products should allow the bacterium to denitrify nitrite to nitrous oxide. We show that N. meningitidis can support growth microaerobically by the denitrification of nitrite via NO and that norB is required for anaerobic growth with nitrite. NorB and, to a lesser extent, the cycP gene product cytochrome c' are able to counteract toxicity due to exogenously added NO. Expression of these genes by N. meningitidis during colonization and disease may confer protection against exogenous or endogenous nitrosative stress.
Figures

References
-
- Berks, B. C., S. J. Ferguson, J. W. Moir, and D. J. Richardson. 1995. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232:97-173. - PubMed
-
- Blondiau, C., P. Lagadec, P. Lejeune, N. Onier, J. M. Cavaillon, and J. F. Jeannin. 1994. Correlation between the capacity to activate macrophages in vitro and the antitumor activity in vivo of lipopolysaccharides from different bacterial species. Immunobiology 190:243-254. - PubMed
-
- Boje, K. M. 1996. Inhibition of nitric oxide synthase attenuates blood-brain barrier disruption during experimental meningitis. Brain Res. 720:75-83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

