Characterization of interactions between the transcriptional repressor PhlF and its binding site at the phlA promoter in Pseudomonas fluorescens F113
- PMID: 12003942
- PMCID: PMC135055
- DOI: 10.1128/JB.184.11.3008-3016.2002
Characterization of interactions between the transcriptional repressor PhlF and its binding site at the phlA promoter in Pseudomonas fluorescens F113
Abstract
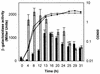
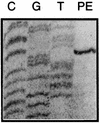
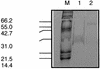
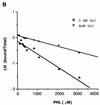
The phlACBD genes responsible for the biosynthesis of the antifungal metabolite 2,4-diacetylphloroglucinol (PHL) by the biocontrol strain Pseudomonas fluorescens F113 are regulated at the transcriptional level by the pathway-specific repressor PhlF. Strong evidence suggests that this regulation occurs mainly in the early logarithmic phase of growth. First, the expression of the phlF gene is relatively high between 3 and 13 h of growth and relatively low thereafter, with the phlACBD operon following an opposite expression profile. Second, the kinetics of PHL biosynthesis are specifically altered in the logarithmic phase in a P. fluorescens F113 phlF mutant. The phlA-phlF intergenic region presents a complex organization in that phlACBD is transcribed from a sigma(70) RNA polymerase-dependent promoter that is likely to overlap the promoter of the divergently transcribed phlF gene. The repression by PhlF is due to its interaction with an inverted repeated sequence, phO, located downstream of the phlA transcriptional start site. Cross-linking experiments indicate that PhlF can dimerize in solution, and thus PhlF may bind phO as a dimer or higher-order complex. Furthermore, it is now demonstrated that certain regulators of PHL synthesis act by modulating PhlF binding to phO. PHL, which has previously been shown to be an autoinducer of PHL biosynthesis, interacts with PhlF to destabilize the PhlF-phO complex. Conversely, the PhlF-phO complex is stabilized by the presence of salicylate, which has been shown to be an inhibitor of phlA expression.
Figures




References
-
- Backes, H., C. Berens, V. Helbl, S. Walter, F. X. Schmid, and W. Hillen. 1997. Combinations of the alpha-helix-turn-alpha-helix motif of TetR with respective residues from LacI or 434Cro: DNA recognition, inducer binding, and urea-dependent denaturation. Biochemistry 36:5311-5322. - PubMed
-
- Bangera, M. G., and L. S. Thomashow. 1996. Characterization of a genomic locus required for synthesis of the antibiotic 2,4-diacetylphloroglucinol by the biological control agent Pseudomonas fluorescens Q2-87. Mol. Plant-Microbe Interact. 9:83-90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

