Stromal effects on mammary gland development and breast cancer
- PMID: 12004111
- PMCID: PMC2788989
- DOI: 10.1126/science.1067431
Stromal effects on mammary gland development and breast cancer
Abstract
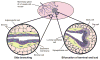
Breast cancer manifests itself in the mammary epithelium, yet there is a growing recognition that mammary stromal cells also play an important role in tumorigenesis. During its developmental cycle, the mammary gland displays many of the properties associated with breast cancer, and many of the stromal factors necessary for mammary development also promote or protect against breast cancer. Here we review our present knowledge of the specific factors and cell types that contribute to epithelial-stromal crosstalk during mammary development. To find cures for diseases like breast cancer that rely on epithelial-stromal crosstalk, we must understand how these different cell types communicate with each other.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

