Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach
- PMID: 12006486
- PMCID: PMC126011
- DOI: 10.1093/emboj/21.10.2332
Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach
Abstract
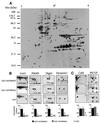
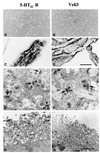
Membrane-bound receptors such as tyrosine kinases and ionotropic receptors are associated with large protein networks structured by protein-protein interactions involving multidomain proteins. Although these networks have emerged as a general mechanism of cellular signalling, much less is known about the protein complexes associated with G-protein-coupled receptors (GPCRs). Using a proteomic approach based on peptide affinity chromatography followed by mass spectrometry and immunoblotting, we have identified 15 proteins that interact with the C- terminal tail of the 5-hydroxytryptamine 2C (5-HT(2C)) receptor, a GPCR. These proteins include several synaptic multidomain proteins containing one or several PDZ domains (PSD95 and the proteins of the tripartite complex Veli3-CASK-Mint1), proteins of the actin/spectrin cytoskeleton and signalling proteins. Coimmunoprecipitation experiments showed that 5-HT(2C) receptors interact with PSD95 and the Veli3-CASK-Mint1 complex in vivo. Electron microscopy also indicated a synaptic enrichment of Veli3 and 5-HT(2C) receptors and their colocalization in microvilli of choroidal cells. These results indicate that the 5-HT(2C) receptor is associated with protein networks that are important for its synaptic localization and its coupling to the signalling machinery.
Figures




References
-
- Abramowski D., Rigo,M., Duc,D., Hoyer,D. and Staufenbiel,M. (1995) Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology, 34, 1635–1645. - PubMed
-
- Bécamel C., Figge,A., Poliak,S., Dumuis,A., Peles,E., Bockaert,J., Lubbert,H. and Ullmer,C. (2001) Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J. Biol. Chem., 276, 12974–12982. - PubMed
-
- Biederer T. and Südhof,T.C. (2000) Mints as adaptors. Direct binding to neurexins and recruitment of Munc18. J. Biol. Chem., 275, 39803–39806. - PubMed
-
- Biederer T. and Südhof,T.C. (2001) Cask and protein 4.1 support F-actin nucleation on neurexins. J. Biol. Chem., 276, 47869–47876. - PubMed
-
- Borg J.P., Straight,S.W., Kaech,S.M., de Taddeo-Borg,M., Kroon,D.E., Karnak,D., Turner,R.S., Kim,S.K. and Margolis,B. (1998) Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem., 273, 31633–31636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

