Essential role of calcineurin in response to endoplasmic reticulum stress
- PMID: 12006487
- PMCID: PMC126012
- DOI: 10.1093/emboj/21.10.2343
Essential role of calcineurin in response to endoplasmic reticulum stress
Abstract
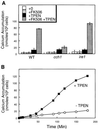
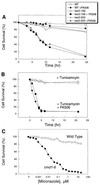
Depletion of calcium ions (Ca2+) from the endoplasmic reticulum (ER) of yeast cells resulted in the activation of the unfolded protein response (UPR) signaling pathway involving Ire1p and Hac1p. The depleted ER also stimulated Ca2+ influx at the plasma membrane through the Cch1p-Mid1p Ca2+ channel and another system. Surprisingly, both Ca2+ influx systems were stimulated upon accumulation of misfolded proteins in the ER even in the presence of Ca2+. The ability of misfolded ER proteins to stimulate Ca2+ influx at the plasma membrane did not require Ire1p or Hac1p, and Ca2+ influx and signaling factors were not required for initial UPR signaling. However, activation of the Ca2+ channel, calmodulin, calcineurin and other factors was necessary for long-term survival of cells undergoing ER stress. A similar calcium cell survival (CCS) pathway operates in the pathogenic fungi and promotes resistance to azole antifungal drugs. These findings reveal an unanticipated new regulatory mechanism that couples ER stress to Ca2+ influx and signaling pathways, which help to prevent cell death and promote resistance to an important class of fungistatic drugs.
Figures




References
-
- Brachmann C.B., Davies,A., Cost,G.J., Caputo,E., Li,J., Hieter,P. and Boeke,J.D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast, 14, 115–132. - PubMed
-
- Burk S.E., Lytton,J., MacLennan,D.H. and Shull,G.E. (1989) cDNA cloning, functional expression and mRNA tissue distribution of a third organellar Ca2+ pump. J. Biol. Chem., 264, 18561–18568. - PubMed
-
- Carnero E., Ribas,J.C., Garcia,B., Duran,A. and Sanchez,Y. (2000) Schizosaccharomyces pombe ehs1p is involved in maintaining cell wall integrity and in calcium uptake. Mol. Gen. Genet., 264, 173–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

