Selective and signal-dependent recruitment of membrane proteins to secretory granules formed by heterologously expressed von Willebrand factor
- PMID: 12006654
- PMCID: PMC111128
- DOI: 10.1091/mbc.01-09-0462
Selective and signal-dependent recruitment of membrane proteins to secretory granules formed by heterologously expressed von Willebrand factor
Abstract
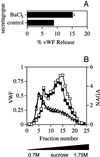
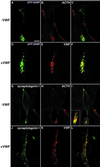
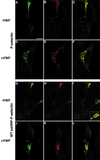
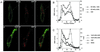
von Willebrand factor (vWF) is a large, multimeric protein secreted by endothelial cells and involved in hemostasis. When expressed in AtT-20 cells, vWF leads to the de novo formation of cigar-shaped organelles similar in appearance to the Weibel-Palade bodies of endothelial cells in which vWF is normally stored before regulated secretion. The membranes of this vWF-induced organelle, termed the pseudogranule, are uncharacterized. We have examined the ability of these pseudogranules, which we show are secretagogue responsive, to recruit membrane proteins. Coexpression experiments show that the Weibel-Palade body proteins P-selectin and CD63, as well as the secretory organelle membrane proteins vesicle-associated membrane protein-2 and synaptotagmin I are diverted away from the endogenous adrenocorticotropic hormone-containing secretory granules to the vWF-containing pseudogranules. However, transferrin receptor, lysosomal-associated membrane protein 1, and sialyl transferase are not recruited. The recruitment of P-selectin is dependent on a tyrosine-based motif within its cytoplasmic domain. Our data show that vWF pseudogranules specifically recruit a subset of membrane proteins, and that in a process explicitly driven by the pseudogranule content (i.e., vWF), the active recruitment of at least one component of the pseudogranule membrane (i.e., P-selectin) is dependent on residues of P-selectin that are cytosolic and therefore unable to directly interact with vWF.
Figures




References
-
- Blagoveshchenskaya AD, Cutler DF. Biochemical analyses of trafficking with horseradish peroxidase-tagged chimeras. Methods Enzymol. 2000a;327:45–60. - PubMed
-
- Blagoveshchenskaia AD, Kornilova ES, Nikol'skii NN. The endocytosis of the epidermal growth factor (EGF) in EGF-dependent cells of the HC11 mouse mammary epithelium line. Tsitologiia. 1995;37:883–892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

