Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia
- PMID: 12006670
- PMCID: PMC111144
- DOI: 10.1091/mbc.02-02-0017
Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia
Abstract
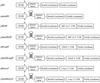
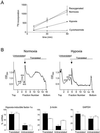
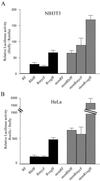
HIF-1alpha is the regulated subunit of the HIF-1 transcription factor, which induces transcription of a number of genes involved in the cellular response to hypoxia. The HIF-1alpha protein is rapidly degraded in cells supplied with adequate oxygen but is stabilized in hypoxic cells. Using polysome profile analysis, we found that translation of HIF-1alpha mRNA in NIH3T3 cells is spared the general reduction in translation rate that occurs during hypoxia. To assess whether the 5'UTR of the HIF-1alpha mRNA contains an internal ribosome entry site (IRES), we constructed a dicistronic reporter with the HIF-1alpha 5'UTR inserted between two reporter coding regions. We found that the HIF-1alpha 5'UTR promoted translation of the downstream reporter, indicating the presence of an IRES. The IRES had activity comparable to that of the well-characterized c-myc IRES. IRES activity was not affected by hypoxic conditions that caused a reduction in cap-dependent translation, and IRES activity was less affected by serum-starvation than was cap-dependent translation. These data indicate that the presence of an IRES in the HIF-1alpha 5'UTR allows translation to be maintained under conditions that are inhibitory to cap-dependent translation.
Figures
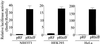

Similar articles
-
Functional integrity of nuclear factor kappaB, phosphatidylinositol 3'-kinase, and mitogen-activated protein kinase signaling allows tumor necrosis factor alpha-evoked Bcl-2 expression to provoke internal ribosome entry site-dependent translation of hypoxia-inducible factor 1alpha.Cancer Res. 2004 Dec 15;64(24):9041-8. doi: 10.1158/0008-5472.CAN-04-1437. Cancer Res. 2004. PMID: 15604270
-
Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia.Mol Cell Biol. 1998 Jun;18(6):3112-9. doi: 10.1128/MCB.18.6.3112. Mol Cell Biol. 1998. PMID: 9584152 Free PMC article.
-
HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression.Mol Cell Biol. 2001 Jun;21(12):3995-4004. doi: 10.1128/MCB.21.12.3995-4004.2001. Mol Cell Biol. 2001. PMID: 11359907 Free PMC article.
-
Characterization of HIF-1 alpha overexpressing HeLa cells and implications for gene therapy.Comp Biochem Physiol C Toxicol Pharmacol. 2002 Dec;133(4):475-81. doi: 10.1016/s1532-0456(02)00117-5. Comp Biochem Physiol C Toxicol Pharmacol. 2002. PMID: 12458176 Review.
-
The role of the 5' untranslated region of an mRNA in translation regulation during development.Int J Biochem Cell Biol. 1999 Jan;31(1):87-106. doi: 10.1016/s1357-2725(98)00134-4. Int J Biochem Cell Biol. 1999. PMID: 10216946 Review.
Cited by
-
eIF4E binding protein 1 expression is associated with clinical survival outcomes in colorectal cancer.Oncotarget. 2015 Sep 15;6(27):24092-104. doi: 10.18632/oncotarget.4483. Oncotarget. 2015. PMID: 26204490 Free PMC article.
-
Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism.Adv Nutr. 2012 May 1;3(3):307-21. doi: 10.3945/an.112.002113. Adv Nutr. 2012. PMID: 22585904 Free PMC article. Review.
-
Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control.EMBO J. 2006 Mar 8;25(5):1114-25. doi: 10.1038/sj.emboj.7600998. Epub 2006 Feb 9. EMBO J. 2006. PMID: 16467844 Free PMC article.
-
Transcriptional repression of hypoxia-inducible factor-1 (HIF-1) by the protein arginine methyltransferase PRMT1.Mol Biol Cell. 2014 Mar;25(6):925-35. doi: 10.1091/mbc.E13-07-0423. Epub 2014 Jan 22. Mol Biol Cell. 2014. PMID: 24451260 Free PMC article.
-
Research progress on the tsRNA classification, function, and application in gynecological malignant tumors.Cell Death Discov. 2021 Dec 14;7(1):388. doi: 10.1038/s41420-021-00789-2. Cell Death Discov. 2021. PMID: 34907180 Free PMC article. Review.
References
-
- Akiri G, Nahari D, Finkelstein Y, Le SY, Elroy-Stein O, Levi BZ. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 1998;17:227–236. - PubMed
-
- Amellem O, Pettersen EO. Cell inactivation and cell cycle inhibition as induced by extreme hypoxia: the possible role of cell cycle arrest as a protection against hypoxia-induced lethal damage. Cell Prolif. 1991;24:127–141. - PubMed
-
- Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem. 1995;270:29083–29089. - PubMed
-
- Gan W, Rhoads RE. Internal initiation of translation directed by the 5′- untranslated region of the mRNA for eIF4G, a factor involved in the picornavirus- induced switch from cap-dependent to internal initiation. J Biol Chem. 1996;271:623–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

