Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens
- PMID: 12010988
- PMCID: PMC127986
- DOI: 10.1128/IAI.70.6.2982-2988.2002
Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens
Abstract
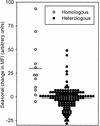
In areas of intense Plasmodium falciparum transmission, protective immunity is acquired during childhood in parallel with acquisition of agglutinating antibodies to parasite-encoded variant surface antigens (VSA) expressed on parasitized red blood cells. In a semi-immune child in such an area, clinical disease is caused mainly by parasites expressing VSA not recognized by preexisting VSA-specific antibodies in that child. Such malaria episodes are known to cause an increase in agglutinating antibodies specifically recognizing VSA expressed by the parasite isolate causing the illness, whereas antibody responses to other parasite isolates are relatively unaffected. However, the detailed kinetics of this VSA antibody acquisition are unknown and hence were the aim of this study. We show that P. falciparum malaria in Ghanaian children generally caused a rapid and sustained increase in variant-specific VSA antibody levels, while more transient and limited increases in levels of antibodies to VSA expressed by other parasite isolates were also seen. Plasma VSA antibody levels were positively correlated with the age of the healthy plasma donors but negatively correlated with the age of the parasite donors (the malaria patient). The data from this first detailed longitudinal study of acquisition of VSA antibodies support the hypothesis that naturally acquired protective immunity to P. falciparum malaria is mediated, at least in part, by VSA-specific antibodies.
Figures




Comment in
-
Understanding naturally acquired immunity to Plasmodium falciparum malaria.Infect Immun. 2003 Feb;71(2):589-90. doi: 10.1128/IAI.71.2.589-590.2003. Infect Immun. 2003. PMID: 12540533 Free PMC article. Review. No abstract available.
References
-
- Afari, E. A., M. Appawu, S. Dunyo, A. Baffoe-Wilmot, and F. K. Nkrumah. 1995. Malaria infection, morbidity and transmission in two ecological zones in southern Ghana. Afr. J. Health Sci. 2:312-316. - PubMed
-
- Afari, E. A., K. A. Koram, S. K. Dunyo, and F. K. Nkrumah. 1992. The epidemiology of malaria with special emphasis on transmission, morbidity, mortality and disease control in Ghana. Internal report. Noguchi Memorial Institute for Medical Research, Legon, Ghana.
-
- Barnwell, J. W., C. Ockenhouse, and D. M. Knowles II. 1985. Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J. Immunol. 135:3494-3497. - PubMed
-
- Baruch, D. I., B. L. Pasloske, H. B. Singh, et al. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

