Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells
- PMID: 12010990
- PMCID: PMC127996
- DOI: 10.1128/IAI.70.6.2995-3003.2002
Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells
Abstract
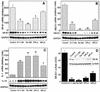
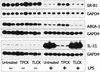
Lipopolysaccharide (LPS) has recently been shown to facilitate macrophage foam cell formation and has been suggested to be a proatherogenic factor. The mechanism of LPS induced cholesterol accumulation, however, is unclear. In this report, using the macrophage-like RAW 264.7 cell line, we provide experimental evidence that LPS's proatherogenic effects may at least in part reflect altered cholesterol metabolism. Data presented demonstrate that in a dose-dependent manner, LPS is able to down regulate the mRNA expression of the two primary high-density lipoprotein (HDL) receptors, scavenger receptor B1 (SR-B1) and ATP binding cassette A1 (ABCA1), with a 50% inhibitory concentration of less than 0.2 ng/ml, as well as to decrease SR-B1 protein expression by 80%. We also found that LPS treatment resulted in a significant decrease (to 20% of the control level) of the specific (125)I-HDL binding as well as in 50% inhibition of the HDL-mediated cholesterol efflux compared to untreated cells. In addition, we compared the potencies of various modified LPS preparations and demonstrated that the phosphorylated lipid A portion of LPS, which is highly conserved among gram-negative microorganisms, including Chlamydia, is primarily responsible for the effects of LPS on SR-B1 and ABCA1 expression. Inhibitors of NF-kappaB activation were observed to efficiently block the suppressive effect of LPS on SR-B1 and ABCA1, suggesting a mechanism involving NF-kappaB. These data indicate that the LPS effects on cholesterol metabolism may contribute to the proatherogenic properties of LPS.
Figures



References
-
- Acton, S. L., A. Rigotti, K. T. Landschulz, S. Xu, H. H. Hobbs, and M. Krieger. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518-520. - PubMed
-
- Baeuerle, P. A., and V. R. Baichwal. 1997. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 65:111-137. - PubMed
-
- Bocharov, A. V., T. G. Vishnyakova, I. N. Baranova, A. P. Patterson, and T. L. Eggerman. 2001. Characterization of a 95 kDa high affinity human high density lipoprotein-binding protein. Biochemistry 140:4407-4416. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Buechler, C., M. Ritter, C. D. Quoc, A. Agildere, and G. Schmitz. 1999. Lipopolysaccharide inhibits the expression of the scavenger receptor Cla-1 in human monocytes and macrophages. Biochem. Biophys. Res. Commun. 262:251-254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

