Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria
- PMID: 12010996
- PMCID: PMC128028
- DOI: 10.1128/IAI.70.6.3040-3052.2002
Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria
Abstract
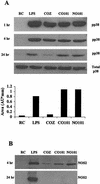
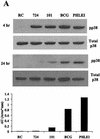
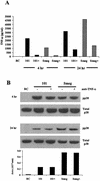
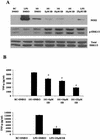
Mycobacteria are the etiologic agents of numerous diseases which account for significant morbidity and mortality in humans and other animal species. Many mycobacteria are intramacrophage pathogens and therefore the macrophage response to infection, which includes synthesis of cytokines such as tumor necrosis factor alpha (TNF-alpha) and production of nitric oxide, has important consequences for host immunity. However, very little is known about the macrophage cell signaling pathways initiated upon infection or how pathogenic mycobacteria may modulate the macrophage responses. Using primary murine bone marrow macrophages, we established that p38 and extracellular signal-regulated kinases 1 and 2 of the mitogen-activated protein kinase (MAPK) pathways are activated upon infection with different species of mycobacteria. However, we observed decreased MAPK activity over time in macrophages infected with pathogenic Mycobacterium avium strains relative to infections with nonpathogenic mycobacteria. Furthermore, macrophages infected with M. avium produced lower levels of TNF-alpha, interleukin 1beta, and inducible nitric oxide synthase 2 than macrophages infected with nonpathogenic species. Inhibitor studies indicate that the MAPKs are required for the Mycobacterium-mediated induction of these effector proteins. Our data indicate that MAPKs are activated in macrophages upon invasion by mycobacteria and that this activation is diminished in macrophages infected with pathogenic strains of M. avium, resulting in decreased production of important immune effector proteins. The decreased MAPK activation associated with M. avium infections suggests a novel point of immune intervention by this mycobacterial species.
Figures







Similar articles
-
Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways.J Immunol. 2004 May 1;172(9):5588-97. doi: 10.4049/jimmunol.172.9.5588. J Immunol. 2004. PMID: 15100302
-
Differential activation of the transcription factor cyclic AMP response element binding protein (CREB) in macrophages following infection with pathogenic and nonpathogenic mycobacteria and role for CREB in tumor necrosis factor alpha production.Infect Immun. 2005 Jan;73(1):514-22. doi: 10.1128/IAI.73.1.514-522.2005. Infect Immun. 2005. PMID: 15618191 Free PMC article.
-
Activation and mitogen-activated protein kinase regulation of transcription factors Ets and NF-kappaB in Mycobacterium-infected macrophages and role of these factors in tumor necrosis factor alpha and nitric oxide synthase 2 promoter function.Infect Immun. 2005 Oct;73(10):6499-507. doi: 10.1128/IAI.73.10.6499-6507.2005. Infect Immun. 2005. PMID: 16177323 Free PMC article.
-
Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way.Cell Microbiol. 2003 Mar;5(3):133-42. doi: 10.1046/j.1462-5822.2003.00263.x. Cell Microbiol. 2003. PMID: 12614457 Review.
-
The molecular basis and downstream immune consequences of mycobacteria-host cell interactions.FEMS Microbiol Rev. 2023 Mar 10;47(2):fuad009. doi: 10.1093/femsre/fuad009. FEMS Microbiol Rev. 2023. PMID: 36948590 Review.
Cited by
-
TGF-β-mediated sustained ERK1/2 activity promotes the inhibition of intracellular growth of Mycobacterium avium in epithelioid cells surrogates.PLoS One. 2011;6(6):e21465. doi: 10.1371/journal.pone.0021465. Epub 2011 Jun 22. PLoS One. 2011. PMID: 21731758 Free PMC article.
-
Sphingosine kinase-1 (SphK-1) regulates Mycobacterium smegmatis infection in macrophages.PLoS One. 2010 May 17;5(5):e10657. doi: 10.1371/journal.pone.0010657. PLoS One. 2010. PMID: 20498849 Free PMC article.
-
The Caenorhabditis elegans p38 MAPK Gene plays a key role in protection from mycobacteria.Microbiologyopen. 2016 Jun;5(3):436-52. doi: 10.1002/mbo3.341. Epub 2016 Feb 25. Microbiologyopen. 2016. PMID: 26919641 Free PMC article.
-
Striking the right immunological balance prevents progression of tuberculosis.Inflamm Res. 2017 Dec;66(12):1031-1056. doi: 10.1007/s00011-017-1081-z. Epub 2017 Jul 15. Inflamm Res. 2017. PMID: 28711989 Review.
-
Mycobacterium tuberculosis PE_PGRS17 promotes the death of host cell and cytokines secretion via Erk kinase accompanying with enhanced survival of recombinant Mycobacterium smegmatis.J Interferon Cytokine Res. 2013 Aug;33(8):452-8. doi: 10.1089/jir.2012.0083. Epub 2013 May 10. J Interferon Cytokine Res. 2013. PMID: 23663047 Free PMC article.
References
-
- Adams, J. L., and C. J. Czuprynski. 1994. Mycobacterial cell wall components induce the production of TNF-alpha, IL-1, and IL-6 by bovine monocytes and the murine macrophage cell line RAW 264.7. Microb. Pathog. 16:401-411. - PubMed
-
- Ajizian, S. J., B. K. English, and E. A. Meals. 1999. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J. Infect. Dis. 179:939-944. - PubMed
-
- Balcewicz-Sablinska, M. K., J. Keane, H. Kornfeld, and H. G. Remold. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 161:2636-2641. - PubMed
-
- Bellmann, K., V. Burkart, J. Bruckhoff, H. Kolb, and J. Landry. 2000. p38-dependent enhancement of cytokine-induced nitric-oxide synthase gene expression by heat shock protein 70. J. Biol. Chem. 275:18172-18179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

