Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli
- PMID: 12011004
- PMCID: PMC127972
- DOI: 10.1128/IAI.70.6.3101-3110.2002
Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli
Abstract
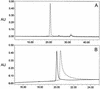
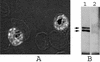
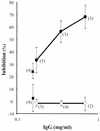
The apical membrane antigen 1 (AMA1) has emerged as a promising vaccine candidate against malaria. Advanced evaluation of its protective efficacy in humans requires the production of highly purified and correctly folded protein. We describe here a process for the expression, fermentation, refolding, and purification of the recombinant ectodomain of AMA1 (amino acids 83(Gly) to 531(Glu)) of Plasmodium falciparum (3D7) produced in Escherichia coli. A synthetic gene containing an E. coli codon bias was cloned into a modified pET32 plasmid, and the recombinant protein was produced by using a redox-modified E. coli strain, Origami (DE3). A purification process was developed that included Sarkosyl extraction followed by affinity purification on a Ni-nitrilotriacetic acid column. The recombinant AMA1 was refolded in the presence of reduced and oxidized glutathione and further purified by using two ion-exchange chromatographic steps. The final product, designated AMA1/E, was homogeneous, monomeric, and >99% pure and had low endotoxin content and low host cell contamination. Analysis of AMA1/E showed that it had the predicted primary sequence, and tertiary structure analysis confirmed its compact disulfide-bonded nature. Rabbit antibodies made to the protein recognized the native parasite AMA1 and inhibited the growth of the P. falciparum homologous 3D7 clone in an in vitro assay. Reduction-sensitive epitopes on AMA1/E were shown to be necessary for the production of inhibitory anti-AMA1 antibodies. AMA1/E was recognized by a conformation-dependent, growth-inhibitory monoclonal antibody, 4G2dc1. The process described here was successfully scaled up to produce AMA1/E protein under GMP conditions, and the product was found to induce highly inhibitory antibodies in rabbits.
Figures

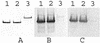
References
-
- Anders, R. F., P. E. Crewther, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunization with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. - PubMed
-
- Burgess, R. R. 1996. Purification of over produced E. coli RNA polymerase σ factors by solubilizing inclusion bodies and refolding from sarkosyl. Methods Enzymol. 273:145-149. - PubMed
-
- Cheng, Q., and A. Saul. 1994. Sequence analysis of the apical membrane antigen 1 (AMA-1) of Plasmodium vivax. Mol. Biochem. Parasitol. 65:183-187. - PubMed
-
- Collins, W. E., D. Pye, P. E. Crewther, K. L. Vandenberg, G. G. Galland, A. J. Sulzer, D. J. Kemp, S. J. Edwards, R. L. Coppel, J. S. Sullivan, et al. 1994. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am. J. Trop. Med. Hyg. 51:711-719. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

