Oral vaccination with subunit vaccines protects animals against aerosol infection with Mycobacterium tuberculosis
- PMID: 12011005
- PMCID: PMC127958
- DOI: 10.1128/IAI.70.6.3111-3121.2002
Oral vaccination with subunit vaccines protects animals against aerosol infection with Mycobacterium tuberculosis
Abstract
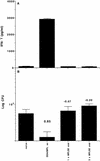
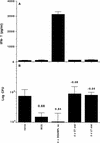
Immunity against Mycobacterium tuberculosis depends largely on activation of cell-mediated responses, and gamma interferon has been shown to play a crucial role in this process in both humans and animal models. Since the lung is normally the organ in which infection is initiated and is the major site of pathology, immune responses in the lung play a significant role in restricting initial infection with M. tuberculosis. The aim of the present study was to stimulate efficient immunity in the lung by targeting the gut mucosa. Detoxified monophosphoryl lipid A (MPL) has been shown to be a relatively nontoxic adjuvant which efficiently promotes the induction of type 1 responses when it is given by the traditional subcutaneous route. We have therefore compared subcutaneous immunization of mice to oral immunization by using a model subunit vaccine carrying two immunodominant proteins from M. tuberculosis, in combination with MPL-based adjuvants. While less effective when used to prime a response, a heterologous priming and boosting vaccination strategy employing oral boosting induced significant systemic type 1 responses which equaled and surpassed those attained by subcutaneous immunization protocols. Moreover, the increased immune responses observed correlated with the induction of substantial protection against subsequent aerosol infection with virulent M. tuberculosis at levels comparable to, or better than, those obtained by multiple subcutaneous vaccinations. These results demonstrate that booster vaccinations via mucosal surfaces, by combining efficient subunit vaccines with the potent adjuvant MPL, may be an effective method of addressing some of the shortcomings of current vaccination strategies.
Figures

Similar articles
-
Characterisation of a live Salmonella vaccine stably expressing the Mycobacterium tuberculosis Ag85B-ESAT6 fusion protein.Vaccine. 2009 Nov 16;27(49):6894-904. doi: 10.1016/j.vaccine.2009.09.007. Epub 2009 Sep 13. Vaccine. 2009. PMID: 19755145 Free PMC article.
-
Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity.J Immunol. 2006 Nov 1;177(9):6353-60. doi: 10.4049/jimmunol.177.9.6353. J Immunol. 2006. PMID: 17056566
-
Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein.Clin Dev Immunol. 2011;2011:617892. doi: 10.1155/2011/617892. Epub 2011 Feb 27. Clin Dev Immunol. 2011. PMID: 21461375 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Mycobacterium tuberculosis proteins involved in cell wall lipid biosynthesis improve BCG vaccine efficacy in a murine TB model.Int J Infect Dis. 2017 Mar;56:274-282. doi: 10.1016/j.ijid.2017.01.024. Epub 2017 Feb 2. Int J Infect Dis. 2017. PMID: 28161464 Review.
Cited by
-
Characterisation of a live Salmonella vaccine stably expressing the Mycobacterium tuberculosis Ag85B-ESAT6 fusion protein.Vaccine. 2009 Nov 16;27(49):6894-904. doi: 10.1016/j.vaccine.2009.09.007. Epub 2009 Sep 13. Vaccine. 2009. PMID: 19755145 Free PMC article.
-
Bridging the gaps to overcome major hurdles in the development of next-generation tuberculosis vaccines.Front Immunol. 2023 Aug 11;14:1193058. doi: 10.3389/fimmu.2023.1193058. eCollection 2023. Front Immunol. 2023. PMID: 37638056 Free PMC article. Review.
-
Oral vaccination with recombinant Lactobacillus casei expressing Aeromonas hydrophila Aha1 against A. hydrophila infections in common carps.Virulence. 2022 Dec;13(1):794-807. doi: 10.1080/21505594.2022.2063484. Virulence. 2022. PMID: 35499101 Free PMC article.
-
Vaccines for tuberculosis: novel concepts and recent progress.Clin Microbiol Rev. 2005 Oct;18(4):687-702. doi: 10.1128/CMR.18.4.687-702.2005. Clin Microbiol Rev. 2005. PMID: 16223953 Free PMC article. Review.
-
Use of Soluble Extracellular Regions of MmpL (SERoM) as Vaccines for Tuberculosis.Sci Rep. 2018 Apr 4;8(1):5604. doi: 10.1038/s41598-018-23893-3. Sci Rep. 2018. PMID: 29618733 Free PMC article.
References
-
- Akbari, O., R. H. DeKruyff, and D. T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725-731. - PubMed
-
- Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22:160-168. - PubMed
-
- Anonymous. 2001. Global tuberculosis control. World Health Organization report W. H. O./CDS/TB/2001.287.
-
- Anonymous. 1995. Global tuberculosis programme and global programme on vaccines: statement on BCG revaccination for the prevention of tuberculosis. Wkly. Epidemiol. Rec. 70: 229-236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

