Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities
- PMID: 12011007
- PMCID: PMC127959
- DOI: 10.1128/IAI.70.6.3130-3142.2002
Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities
Abstract
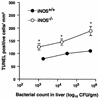
Host defense functions of nitric oxide (NO) are known for many bacterial infections. In this study, we investigated the antimicrobial effect of NO in murine salmonellosis by using inducible NO synthase (iNOS)-deficient mice infected with an avirulent or virulent Salmonella enterica serovar Typhimurium strain. All iNOS-deficient mice died of severe septicemia within 6 days after intraperitoneal injection with an avirulent strain (LT2) to which wild-type mice were highly resistant; 50% lethal doses (LD(50)s) of the LT2 strain for iNOS-deficient and wild-type mice were 30 CFU and 7 x 10(4) CFU, respectively. Lack of NO production in iNOS-deficient mice was verified directly by electron spin resonance spectroscopy. Bacterial yields in liver and blood were much higher in iNOS-deficient mice than in wild-type mice throughout the course of infection. Very small amounts of a virulent strain of serovar Typhimurium (a clinical isolate, strain Gifu 12142; LD(50), 50 CFU) given orally caused severe septicemia in iNOS-deficient animals; wild-type mice tolerated higher doses (LD(50), 6 x 10(2) CFU). Histopathology of livers from infected iNOS-deficient mice revealed extensive damage, such as diffuse hepatocellular apoptosis and increased neutrophil infiltration, but livers from infected wild-type mice showed a limited number of microabscesses, consisting of polymorphonuclear cells and macrophages and low levels of apoptotic change. The LT2 strain was much more susceptible to the bactericidal effect of peroxynitrite than the Gifu strain, suggesting that peroxynitrite resistance may contribute to Salmonella pathogenicity. These results indicate that NO has significant host defense functions in Salmonella infections not only because of its direct antimicrobial effect but also via cytoprotective actions for infected host cells, possibly through its antiapoptotic effect.
Figures

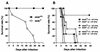
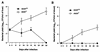
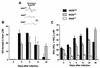



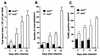

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

