Identification of a Neospora caninum microneme protein (NcMIC1) which interacts with sulfated host cell surface glycosaminoglycans
- PMID: 12011014
- PMCID: PMC127992
- DOI: 10.1128/IAI.70.6.3187-3198.2002
Identification of a Neospora caninum microneme protein (NcMIC1) which interacts with sulfated host cell surface glycosaminoglycans
Abstract
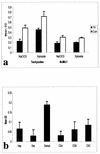
The invasive stages of apicomplexan parasites enter their host cells through mechanisms which are largely conserved throughout the phylum. Host cell invasion is divided into two distinct events, namely, adhesion onto the host cell surface and the actual host cell entry process. The former is mediated largely through microneme proteins which are secreted at the onset of establishing contact with the host cell surface. Many of the microneme proteins identified so far contain adhesive domains. We here present the genomic and corresponding cDNA sequences coding for a 460-amino-acid (aa) microneme protein in Neospora caninum tachyzoites which, due to its homology to MIC1 in Toxoplasma gondii (TgMIC1), was named NcMIC1. The deduced NcMIC1 polypeptide sequence contains an N-terminal signal peptide of 20 aa followed by two tandemly internal repeats of 48 and 44 aa, respectively. Integrated into each repeat is a CXXXCG sequence motif reminiscent of the thrombospondin-related family of adhesive proteins. The positioning of this motif is strictly conserved in TgMIC1 and NcMIC1. The C-terminal part, comprised of 278 aa, was expressed in Escherichia coli, and antibodies affinity purified on recombinant NcMIC1 were used to confirm the localization within the micronemes by immunofluorescence and immunogold transmission electron microscopy of tachyzoites. Immunohistochemistry of mouse brains infected with tissue cysts showed that expression of this protein is reduced in the bradyzoite stage. Upon initiation of secretion by elevating the temperature to 37 degrees C, NcMIC1 is released into the medium supernatant. NcMIC1 binds to trypsinized, rounded Vero cells, as well as to Vero cell monolayers. Removal of glycosaminoglycans from the host cell surface and modulation of host cell surface glycosaminoglycan sulfation significantly reduces the binding of NcMIC1 to the host cell surface. Solid-phase binding assays employing defined glycosaminoglycans confirmed that NcMIC1 binds to sulfated glycosaminoglycans.
Figures
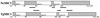






Similar articles
-
Neospora caninum microneme protein NcMIC3: secretion, subcellular localization, and functional involvement in host cell interaction.Infect Immun. 2001 Oct;69(10):6483-94. doi: 10.1128/IAI.69.10.6483-6494.2001. Infect Immun. 2001. PMID: 11553593 Free PMC article.
-
Vero cell surface proteoglycan interaction with the microneme protein NcMIC(3) mediates adhesion of Neospora caninum tachyzoites to host cells unlike that in Toxoplasma gondii.Int J Parasitol. 2002 Jun;32(6):695-704. doi: 10.1016/s0020-7519(02)00014-0. Int J Parasitol. 2002. PMID: 12062488
-
Members of a novel protein family containing microneme adhesive repeat domains act as sialic acid-binding lectins during host cell invasion by apicomplexan parasites.J Biol Chem. 2010 Jan 15;285(3):2064-76. doi: 10.1074/jbc.M109.060988. Epub 2009 Nov 9. J Biol Chem. 2010. PMID: 19901027 Free PMC article.
-
Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii.Int J Parasitol. 2001 Oct;31(12):1293-302. doi: 10.1016/s0020-7519(01)00257-0. Int J Parasitol. 2001. PMID: 11566297 Review.
-
EtMIC4: a microneme protein from Eimeria tenella that contains tandem arrays of epidermal growth factor-like repeats and thrombospondin type-I repeats.Int J Parasitol. 2001 Oct;31(12):1303-10. doi: 10.1016/s0020-7519(01)00255-7. Int J Parasitol. 2001. PMID: 11566298 Review.
Cited by
-
In vitro induction of Neospora caninum bradyzoites in vero cells reveals differential antigen expression, localization, and host-cell recognition of tachyzoites and bradyzoites.Infect Immun. 2004 Jan;72(1):576-83. doi: 10.1128/IAI.72.1.576-583.2004. Infect Immun. 2004. PMID: 14688139 Free PMC article.
-
Unravelling the Neospora caninum secretome through the secreted fraction (ESA) and quantification of the discharged tachyzoite using high-resolution mass spectrometry-based proteomics.Parasit Vectors. 2013 Nov 23;6(1):335. doi: 10.1186/1756-3305-6-335. Parasit Vectors. 2013. PMID: 24267406 Free PMC article.
-
Review of neosporosis: Disease insights and control approaches.Open Vet J. 2025 Mar;15(3):1078-1090. doi: 10.5455/OVJ.2025.v15.i3.2. Epub 2025 Mar 31. Open Vet J. 2025. PMID: 40276172 Free PMC article. Review.
-
Identification and characterization of a microneme protein (NcMIC6) in Neospora caninum.Parasitol Res. 2015 Aug;114(8):2893-902. doi: 10.1007/s00436-015-4490-3. Epub 2015 May 10. Parasitol Res. 2015. PMID: 25956399
-
Genome Wide Identification of Mutational Hotspots in the Apicomplexan Parasite Neospora caninum and the Implications for Virulence.Genome Biol Evol. 2018 Sep 1;10(9):2417-2431. doi: 10.1093/gbe/evy188. Genome Biol Evol. 2018. PMID: 30165699 Free PMC article.
References
-
- Bork, P., A. K. Downing, B. Kieffer, and I. D. Campbell. 1996. Structure and distribution of modules in extracellular proteins. Q. Rev. Biophys. 29:119-167. - PubMed
-
- Brydges, S. D., G. D. Sherman, S. Nockemann, A. Loyens, W. Daubener, J. F. Dubremetz, and V. B. Carruthers. 2000. Molecular characterization of TgMIC5, a proteolytically processed antigen secreted from the micronemes of Toxoplasma gondii. Mol. Biochem. Parasitol. 111:51-66. - PubMed
-
- Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114-123. - PubMed
-
- Carruthers, V. B., and L. D. Sibley. 1999. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31:421-428. - PubMed
-
- Carruthers, V. B., Giddings, O. K., and L. D. Sibley. 1999. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1:225-235. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

