Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency
- PMID: 12011337
- PMCID: PMC155870
- DOI: 10.1104/pp.010869
Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency
Abstract
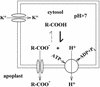
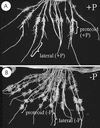
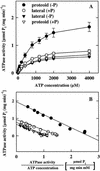
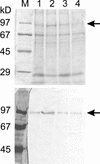
White lupin (Lupinus albus) is able to adapt to phosphorus deficiency by producing proteoid roots that release a huge amount of organic acids, resulting in mobilization of sparingly soluble soil phosphate in rhizosphere. The mechanisms responsible for the release of organic acids by proteoid root cells, especially the trans-membrane transport processes, have not been elucidated. Because of high cytosolic pH, the release of undissociated organic acids is not probable. In the present study, we focused on H+ export by plasma membrane H+ ATPase in active proteoid roots. In vivo, rhizosphere acidification of active proteoid roots was vanadate sensitive. Plasma membranes were isolated from proteoid roots and lateral roots from P-deficient and -sufficient plants. In vitro, in comparison with two types of lateral roots and proteoid roots of P-sufficient plants, the following increase of the various parameters was induced in active proteoid roots of P-deficient plants: (a) hydrolytic ATPase activity, (b) Vmax and Km, (c) H+ ATPase enzyme concentration of plasma membrane, (d) H+-pumping activity, (e) pH gradient across the membrane of plasmalemma vesicles, and (f) passive H+ permeability of plasma membrane. In addition, lower vanadate sensitivity and more acidic pH optimum were determined for plasma membrane ATPase of active proteoid roots. Our data support the hypothesis that in active proteoid root cells, H+ and organic anions are exported separately, and that modification of plasma membrane H+ ATPase is essential for enhanced rhizosphere acidification by active proteoid roots.
Figures



Similar articles
-
A link between citrate and proton release by proteoid roots of white lupin (Lupinus albus L.) grown under phosphorus-deficient conditions?Plant Cell Physiol. 2005 Jun;46(6):892-901. doi: 10.1093/pcp/pci094. Epub 2005 Apr 8. Plant Cell Physiol. 2005. PMID: 15821025
-
Plasma membrane H-ATPase-dependent citrate exudation from cluster roots of phosphate-deficient white lupin.Plant Cell Environ. 2009 May;32(5):465-75. doi: 10.1111/j.1365-3040.2009.01938.x. Epub 2009 Jan 14. Plant Cell Environ. 2009. PMID: 19183296
-
Mobilization and acquisition of sparingly soluble P-Sources by Brassica cultivars under P-starved environment II. Rhizospheric pH changes, redesigned root architecture and pi-uptake kinetics.J Integr Plant Biol. 2009 Nov;51(11):1024-39. doi: 10.1111/j.1744-7909.2009.00873.x. J Integr Plant Biol. 2009. PMID: 19903224
-
Energization of transport processes in plants. roles of the plasma membrane H+-ATPase.Plant Physiol. 2004 Sep;136(1):2475-82. doi: 10.1104/pp.104.048231. Plant Physiol. 2004. PMID: 15375204 Free PMC article. Review. No abstract available.
-
Role of the plasma membrane H(+)-ATPase in the regulation of organic acid exudation under aluminum toxicity and phosphorus deficiency.Plant Signal Behav. 2016;11(1):e1106660. doi: 10.1080/15592324.2015.1106660. Plant Signal Behav. 2016. PMID: 26713714 Free PMC article. Review.
Cited by
-
Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource.New Phytol. 2003 Mar;157(3):423-447. doi: 10.1046/j.1469-8137.2003.00695.x. New Phytol. 2003. PMID: 33873400 Review.
-
The genome evolution and low-phosphorus adaptation in white lupin.Nat Commun. 2020 Feb 26;11(1):1069. doi: 10.1038/s41467-020-14891-z. Nat Commun. 2020. PMID: 32103018 Free PMC article.
-
Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants.Int J Mol Sci. 2021 May 5;22(9):4904. doi: 10.3390/ijms22094904. Int J Mol Sci. 2021. PMID: 34063156 Free PMC article. Review.
-
Citrate-permeable channels in the plasma membrane of cluster roots from white lupin.Plant Physiol. 2004 Nov;136(3):3771-83. doi: 10.1104/pp.104.046201. Epub 2004 Oct 29. Plant Physiol. 2004. PMID: 15516510 Free PMC article.
-
Genetic Engineering of the Biosynthesis of Glycine Betaine Modulates Phosphate Homeostasis by Regulating Phosphate Acquisition in Tomato.Front Plant Sci. 2019 Jan 10;9:1995. doi: 10.3389/fpls.2018.01995. eCollection 2018. Front Plant Sci. 2019. PMID: 30687378 Free PMC article.
References
-
- Baginski ES, Foa PP, Zak B. Determination of phosphate: study of labile organic phosphate interference. Clin Chim Acta. 1967;15:155–158.
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Dellórto M, Santi S, de Nisi P, Cesco S, Varanini Z, Zocchi G, Pinton R. Development of Fe-deficiency responses in cucumber (Cucumis sativus L.) roots: involvement of plasma membrane H+-ATPase activity. J Exp Bot. 2000;51:695–701. - PubMed
-
- DeWitt ND, Harper JF, Sussman MR. Evidence for a plasma membrane proton pump in phloem cells of higher plants. Plant J. 1991;1:121–128. - PubMed
-
- Dinkelaker B, Hengeler C, Marschner H. Distribution and function of proteoid roots and other root clusters. Bot Acta. 1995;108:183–200.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

