Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls
- PMID: 12011351
- PMCID: PMC155884
- DOI: 10.1104/pp.010886
Gibberellin produced in the cotyledon is required for cell division during tissue reunion in the cortex of cut cucumber and tomato hypocotyls
Abstract
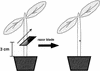
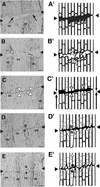
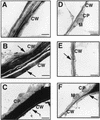
Cucumber (Cucumis sativus) hypocotyls were cut to one-half of their diameter transversely, and morphological and histochemical analyses of the process of tissue reunion in the cortex were performed. Cell division in the cortex commenced 3 d after cutting, and the cortex was nearly fully united within 7 d. 4',6-Diamidino-2-phenylindole staining and 5-bromo-2'-deoxyuridine labeling experiments indicate that nDNA synthesis occurred during this process. In addition, specific accumulation of pectic substances was observed in the cell wall of attached cells in the reunion region of the cortex. Cell division during tissue reunion was strongly inhibited when the cotyledon was removed. This inhibition was reversed by applying gibberellin (GA, 10(-4) M GA3) to the apical tip of the cotyledon-less plant. Supporting this observation, cell division in the cortex was inhibited by treatment of the cotyledon with 10(-4) M uniconazole-P (an inhibitor of GA biosynthesis), and this inhibition was also reversed by simultaneous application of GA. In contrast to the essential role of cotyledon, normal tissue reunion in cut hypocotyls was still observed when the shoot apex was removed. The requirement of GA for tissue reunion in cut hypocotyls was also evident in the GA-deficient gib-1 mutant of tomato (Lycopersicon esculentum). Our results suggest that GA, possibly produced in cotyledons, is essential for cell division in reuniting cortex of cut hypocotyls.
Figures





References
-
- Asamizu T, Nakayama N, Nishi A. Pectic polysaccharides in carrot cells growing in suspension culture. Planta. 1984;160:469–476. - PubMed
-
- Goldberg R, Morvan C, Roland JC. Composition, properties and localization of pectins in young and mature cells of the mung bean hypocotyl. Plant Cell Physiol. 1986;27:417–429.
-
- Groot SPC, Bruinsma J, Karssen CM. The role of endogenous gibberellin in seed and fruit development of tomato: studies with a gibberellin-deficient mutant. Physiol Plant. 1987;71:184–190.
-
- Iwai H, Kikuchi A, Kobayashi T, Kamada H, Satoh S. High levels of non-methylesterified pectins and low levels of peripherally located pectins in loosely attached non-embryogenic callus of carrot. Plant Cell Rep. 1999;18:561–566.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

