Molecular and biochemical characterization of a cold-regulated phosphoethanolamine N-methyltransferase from wheat
- PMID: 12011366
- PMCID: PMC155899
- DOI: 10.1104/pp.001776
Molecular and biochemical characterization of a cold-regulated phosphoethanolamine N-methyltransferase from wheat
Abstract
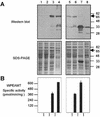
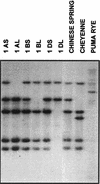
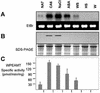
A cDNA that encodes a methyltransferase (MT) was cloned from a cold-acclimated wheat (Triticum aestivum) cDNA library. Molecular analysis indicated that the enzyme WPEAMT (wheat phosphoethanolamine [P-EA] MT) is a bipartite protein with two separate sets of S-adenosyl-L-Met-binding domains, one close to the N-terminal end and the second close to the C-terminal end. The recombinant protein was found to catalyze the three sequential methylations of P-EA to form phosphocholine, a key precursor for the synthesis of phosphatidylcholine and glycine betaine in plants. Deletion and mutation analyses of the two S-adenosyl-L-Met-binding domains indicated that the N-terminal domain could perform the three N-methylation steps transforming P-EA to phosphocholine. This is in contrast to the MT from spinach (Spinacia oleracea), suggesting a different functional evolution for the monocot enzyme. The truncated C-terminal and the N-terminal mutated enzyme were only able to methylate phosphomonomethylethanolamine and phosphodimethylethanolamine, but not P-EA. This may suggest that the C-terminal part is involved in regulating the rate and the equilibrium of the three methylation steps. Northern and western analyses demonstrated that both Wpeamt transcript and the corresponding protein are up-regulated during cold acclimation. This accumulation was associated with an increase in enzyme activity, suggesting that the higher activity is due to de novo protein synthesis. The role of this enzyme during cold acclimation and the development of freezing tolerance are discussed.
Figures



References
-
- Allard F, Houde M, Kröl M, Ivanov A, Huner NPA, Sarhan F. Betaine improves freezing tolerance in wheat. Plant Cell Physiol. 1998;39:1194–1202.
-
- Bohnert H, Jensen RG. Strategies for engineering water stress tolerance in plants. Trends Biotechnol. 1996;14:89–97.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

