Bidirectional cooperative motion of molecular motors
- PMID: 12011432
- PMCID: PMC124465
- DOI: 10.1073/pnas.102692399
Bidirectional cooperative motion of molecular motors
Abstract
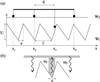
Recently, in a beautiful set of experiments, it has been shown that a Ncd mutant, NK11, which lacks directionality in its individual motion, was able to exhibit a new kind of directed motion in motility assays (Endow, S. A. & Higuchi, H. (2000) Nature (London) 406, 913-916): the filaments keep a given velocity for a while and then suddenly move in the opposite direction with similar velocity. We show that these observations nicely illustrate the concept of dynamic transitions in motor collections introduced earlier in the case of an infinite number of motors. We investigate the experimentally relevant case of a finite number of motors both when directionality is present (kinesins, myosins, Ncd) and absent (NK11). Using a symmetric two-state model, we demonstrate that bidirectional motion is the signature of a dynamic transition that results from the collective behavior of many motors acting on the same filament. For motors exhibiting directional bias individually, an asymmetric two-state model is appropriate. In that case, dynamic transitions exist for motor collections in the presence of an external force. We give predictions for the dependence of motion on ATP concentration, external forces, and the number of motors involved. In particular, we show that the reversal time grows exponentially with the number of motors per filament.
Figures






Comment in
-
Push or pull? Teams of motor proteins have it both ways.Proc Natl Acad Sci U S A. 2002 May 14;99(10):6521-3. doi: 10.1073/pnas.112200199. Proc Natl Acad Sci U S A. 2002. PMID: 12011414 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

