Structure-activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions
- PMID: 12014951
- PMCID: PMC6927247
- DOI: 10.1021/jm0104318
Structure-activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions
Abstract
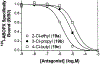
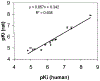
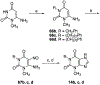
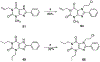
In the search for improved selective antagonist ligands of the A2B adenosine receptor, which have the potential as antiasthmatic or antidiabetic drugs, we have synthesized and screened a variety of alkylxanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions. Competition for 125I-ABOPX (125I-3-(4-amino-3-iodobenzyl)-8-(phenyl-4-oxyacetate)-1-propylxanthine) binding in membranes of stably transfected HEK-293 cells revealed uniformly higher affinity (<10-fold) of these xanthines for human than for rat A2B adenosine receptors. Binding to rat brain membranes expressing A1 and A2A adenosine receptors revealed greater A2B selectivity over A2A than A1 receptors. Substitution at the 1-position with 2-phenylethyl (or alkyl/olefinic groups) and at N-3 with hydrogen or methyl favored A2B selectivity. Relative to enprofylline 2b, pentoxifylline 35 was equipotent and 1-propylxanthine 3 was >13-fold more potent at human A2B receptors. Most N-7 substituents did not enhance affinity over hydrogen, except for 7-(2-chloroethyl), which enhanced the affinity of theophylline by 6.5-fold to 800 nM. The A2B receptor affinity-enhancing effects of 7-(2-chloroethyl) vs 7-methyl were comparable to the known enhancement produced by an 8-aryl substitution. Among 8-phenyl analogues, a larger alkyl group at the 1-position than at the 3-position favored affinity at the human A2B receptor, as indicated by 1-allyl-3-methyl-8-phenylxanthine, with a K(i) value of 37 nM. Substitution on the 8-phenyl ring indicated that an electron-rich ring was preferred for A2B receptor binding. In conclusion, new leads for the design of xanthines substituted in the 1-, 3-, 7-, and 8-positions as A2B receptor-selective antagonists have been identified.
Figures

Similar articles
-
Progress in the pursuit of therapeutic adenosine receptor antagonists.Med Res Rev. 2006 Mar;26(2):131-59. doi: 10.1002/med.20048. Med Res Rev. 2006. PMID: 16380972 Free PMC article. Review.
-
1,8-disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists.J Med Chem. 2002 Mar 28;45(7):1500-10. doi: 10.1021/jm011049y. J Med Chem. 2002. PMID: 11906291
-
Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors.J Med Chem. 2000 Mar 23;43(6):1165-72. doi: 10.1021/jm990421v. J Med Chem. 2000. PMID: 10737749 Free PMC article.
-
Design, synthesis, and biological evaluation of new 8-heterocyclic xanthine derivatives as highly potent and selective human A2B adenosine receptor antagonists.J Med Chem. 2004 Mar 11;47(6):1434-47. doi: 10.1021/jm0309654. J Med Chem. 2004. PMID: 14998332
-
Xanthines as adenosine receptor antagonists.Handb Exp Pharmacol. 2011;(200):151-99. doi: 10.1007/978-3-642-13443-2_6. Handb Exp Pharmacol. 2011. PMID: 20859796 Free PMC article. Review.
Cited by
-
Mechanism of adenosine-induced airways obstruction in allergic guinea pigs.Br J Pharmacol. 2006 Apr;147(7):720-8. doi: 10.1038/sj.bjp.0706663. Br J Pharmacol. 2006. PMID: 16432507 Free PMC article.
-
Aminophylline for renal protection in neonatal hypoxic-ischemic encephalopathy in the era of therapeutic hypothermia.Pediatr Res. 2021 Mar;89(4):974-980. doi: 10.1038/s41390-020-0999-y. Epub 2020 Jun 5. Pediatr Res. 2021. PMID: 32503030 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update.Pharmacol Rev. 2011 Mar;63(1):1-34. doi: 10.1124/pr.110.003285. Epub 2011 Feb 8. Pharmacol Rev. 2011. PMID: 21303899 Free PMC article. Review.
-
Recent improvements in the development of A(2B) adenosine receptor agonists.Purinergic Signal. 2008 Dec;4(4):287-303. doi: 10.1007/s11302-008-9097-z. Epub 2008 Apr 29. Purinergic Signal. 2008. PMID: 18443746 Free PMC article.
-
Progress in the pursuit of therapeutic adenosine receptor antagonists.Med Res Rev. 2006 Mar;26(2):131-59. doi: 10.1002/med.20048. Med Res Rev. 2006. PMID: 16380972 Free PMC article. Review.
References
-
- Linden J; Jacobson KA Molecular biology of recombinant adenosine receptors In Cardiovascular Biology of Purines; Burnstock G, Dobson JG, Liang BT, Linden J, Eds.; Kluwer: Norwell, MA, 1998; pp 1–20.
-
- Feoktistov I; Murray JJ; Biaggioni I Positive modulation of intracellular Ca2+ by adenosine A2B, prostacyclin and prostaglandin E2 receptors via a cholera toxin-sensitive mechanism in human erythroleukemia cells. Mol. Pharmacol 1994, 45, 1160–1167. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

