Mitochondria from cultured cells derived from normal and thiamine-responsive megaloblastic anemia individuals efficiently import thiamine diphosphate
- PMID: 12014993
- PMCID: PMC111190
- DOI: 10.1186/1471-2091-3-8
Mitochondria from cultured cells derived from normal and thiamine-responsive megaloblastic anemia individuals efficiently import thiamine diphosphate
Abstract
Background: Thiamine diphosphate (ThDP) is the active form of thiamine, and it serves as a cofactor for several enzymes, both cytosolic and mitochondrial. Isolated mitochondria have been shown to take up thiamine yet thiamine diphosphokinase is cytosolic and not present in mitochondria. Previous reports indicate that ThDP can also be taken up by rat mitochondria, but the kinetic constants associated with such uptake seemed not to be physiologically relevant.
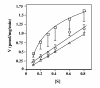
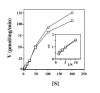
Results: Here we examine ThDP uptake by mitochondria from several human cell types, including cells from patients with thiamine-responsive megaloblastic anemia (TRMA) that lack a functional thiamine transporter of the plasma membrane. Although mitochondria from normal lymphoblasts took up thiamine in the low micromolar range, surprisingly mitochondria from TRMA lymphoblasts lacked this uptake component. ThDP was taken up efficiently by mitochondria isolated from either normal or TRMA lymphoblasts. Uptake was saturable and biphasic with a high affinity component characterized by a Km of 0.4 to 0.6 microM. Mitochondria from other cell types possessed a similar high affinity uptake component with variation seen in uptake capacity as revealed by differences in Vmax values.
Conclusions: The results suggest a shared thiamine transporter for mitochondria and the plasma membrane. Additionally, a high affinity component of ThDP uptake by mitochondria was identified with the apparent affinity constant less than the estimates of the cytosolic concentration of free ThDP. This finding indicates that the high affinity uptake is physiologically significant and may represent the main mechanism for supplying phosphorylated thiamine for mitochondrial enzymes.
Figures


Similar articles
-
Defective high-affinity thiamine transporter leads to cell death in thiamine-responsive megaloblastic anemia syndrome fibroblasts.J Clin Invest. 1999 Mar;103(5):723-9. doi: 10.1172/JCI3895. J Clin Invest. 1999. PMID: 10074490 Free PMC article.
-
Thiamine-responsive megaloblastic anemia syndrome (TRMA) with cone-rod dystrophy.Ophthalmic Genet. 2000 Dec;21(4):243-50. Ophthalmic Genet. 2000. PMID: 11135496
-
Cardiac manifestations in thiamine-responsive megaloblastic anemia syndrome.Pediatr Cardiol. 2003 Sep-Oct;24(5):476-81. doi: 10.1007/s00246-002-0215-3. Pediatr Cardiol. 2003. PMID: 14627317 Review.
-
Novel mutation in the SLC19A2 gene in an Iranian family with thiamine-responsive megaloblastic anemia: a series of three cases.J Clin Res Pediatr Endocrinol. 2013 Sep 10;5(3):199-201. doi: 10.4274/Jcrpe.969. J Clin Res Pediatr Endocrinol. 2013. PMID: 24072090 Free PMC article.
-
Thiamine-responsive megaloblastic anemia syndrome: a disorder of high-affinity thiamine transport.Blood Cells Mol Dis. 2001 Jan-Feb;27(1):135-8. doi: 10.1006/bcmd.2000.0356. Blood Cells Mol Dis. 2001. PMID: 11358373 Review.
Cited by
-
Presence of thiamine pyrophosphate in mammalian peroxisomes.BMC Biochem. 2007 Jun 27;8:10. doi: 10.1186/1471-2091-8-10. BMC Biochem. 2007. PMID: 17596263 Free PMC article.
-
Monogenic syndromes of abnormal glucose homeostasis: clinical review and relevance to the understanding of the pathology of insulin resistance and beta cell failure.J Med Genet. 2005 Dec;42(12):893-902. doi: 10.1136/jmg.2005.030791. Epub 2005 Mar 16. J Med Genet. 2005. PMID: 15772126 Free PMC article. Review.
-
Uncovering the beginning of diabetes: the cellular redox status and oxidative stress as starting players in hyperglycemic damage.Mol Cell Biochem. 2013 Apr;376(1-2):103-10. doi: 10.1007/s11010-012-1555-9. Epub 2013 Jan 8. Mol Cell Biochem. 2013. PMID: 23292031
-
Single-molecule FRET studies on the cotranscriptional folding of a thiamine pyrophosphate riboswitch.Proc Natl Acad Sci U S A. 2018 Jan 9;115(2):331-336. doi: 10.1073/pnas.1712983115. Epub 2017 Dec 26. Proc Natl Acad Sci U S A. 2018. PMID: 29279370 Free PMC article.
-
High dose thiamine improves fatigue in multiple sclerosis.BMJ Case Rep. 2013 Jul 16;2013:bcr2013009144. doi: 10.1136/bcr-2013-009144. BMJ Case Rep. 2013. PMID: 23861280 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

