Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses
- PMID: 12015438
- PMCID: PMC2290309
- DOI: 10.1113/jphysiol.2002.016857
Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses
Abstract
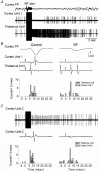
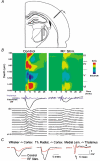
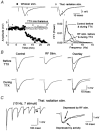
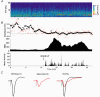
The thalamus serves as a gate that regulates the flow of sensory inputs to the neocortex, and this gate is controlled by neuromodulators from the brainstem reticular formation that are released during arousal. Here we show in rats that sensory-evoked responses were suppressed in the neocortex by activating the brainstem reticular formation and during natural arousal. Sensory suppression occurred at the thalamocortical connection and was a consequence of the activity-dependent depression of thalamocortical synapses caused by increased thalamocortical tonic firing during arousal. Thalamocortical suppression may serve as a mechanism to focus sensory inputs to their appropriate representations in neocortex, which is helpful for the spatial processing of sensory information.
Figures


References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
-
- Armstrong-James M. The nature and plasticity of sensory processing within adult rat barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex, The Barrel Cortex of Rodents. Vol. 11. New York: Plenum; 1995. pp. 333–374.
-
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 ‘barrel’ cortex. Journal of Comparative Neurology. 1987;263:265–281. - PubMed
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. Journal of Neurophysiology. 1992;68:1345–1358. - PubMed
-
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Progress in Brain Research. 1991;88:501–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

