Identification of major glucan-associated cell wall proteins of Candida albicans and their role in fluconazole resistance
- PMID: 12019077
- PMCID: PMC127269
- DOI: 10.1128/AAC.46.6.1688-1694.2002
Identification of major glucan-associated cell wall proteins of Candida albicans and their role in fluconazole resistance
Abstract
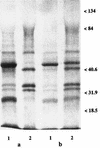
Identification of major glucan-associated proteins (GAPs) of the cell wall of a number of Candida albicans isolates susceptible or resistant to fluconazole (FLC) was addressed by direct sequencing of the protein bands resolved by unidimensional gel electrophoresis. Changes in the GAP compositions of the different strains grown in the presence of the drug were also investigated. In the FLC-susceptible strains, the major (more abundant) GAPs were enolase (46 kDa), two isoforms of phosphoglyceromutase (32 and 29 kDa), and two beta-(1-3)-exoglucanases (44 and 34 kDa), one of which (the 34-kDa component) was glycosylated. When these strains were grown in the presence of FLC there were substantial decreases in the intensities of the two enzymes of the glycolytic pathway (enolase and the phosphoglyceromutases), which were apparently replaced by enhancement of the exoglucanase constituents, particularly the 44-kDa one. This GAP pattern closely mimicked that observed in the FLC-resistant strains whether they were grown in the presence or in the absence of the drug. Both the enolase and the exoglucanase constituents were detected in the culture supernatants of FLC-treated cells, together with substantial amounts of highly glycosylated, probably mannoprotein secretory material, suggesting that FLC may cause marked alterations of GAP incorporation into the cell wall. Altogether, we were able to identify all major GAP constituents and monitor their distributions in the cell wall of C. albicans during treatment with FLC. The near equivalence of the GAP profile for the FLC-susceptible strain grown in the presence of FLC to that for the FLC-resistant strain suggests that the effects of the drug on GAPs may be stably incorporated into the cell wall of the fungus upon acquisition of resistance.
Figures




Similar articles
-
The lipopeptide antimycotic, cilofungin modulates the incorporation of glucan-associated proteins into the cell wall of Candida albicans.J Antimicrob Chemother. 1994 Jun;33(6):1137-46. doi: 10.1093/jac/33.6.1137. J Antimicrob Chemother. 1994. PMID: 7928807
-
Low levels of antigenic variability in fluconazole-susceptible and -resistant Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis.Clin Diagn Lab Immunol. 1999 Sep;6(5):665-70. doi: 10.1128/CDLI.6.5.665-670.1999. Clin Diagn Lab Immunol. 1999. PMID: 10473514 Free PMC article.
-
Antifungal activity of thymol against clinical isolates of fluconazole-sensitive and -resistant Candida albicans.J Med Microbiol. 2009 Aug;58(Pt 8):1074-1079. doi: 10.1099/jmm.0.008052-0. Epub 2009 Jun 15. J Med Microbiol. 2009. PMID: 19528168
-
Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery.Int J Antimicrob Agents. 2014 May;43(5):395-402. doi: 10.1016/j.ijantimicag.2013.12.009. Epub 2014 Jan 22. Int J Antimicrob Agents. 2014. PMID: 24503221 Review.
-
Cell wall and secreted proteins of Candida albicans: identification, function, and expression.Microbiol Mol Biol Rev. 1998 Mar;62(1):130-80. doi: 10.1128/MMBR.62.1.130-180.1998. Microbiol Mol Biol Rev. 1998. PMID: 9529890 Free PMC article. Review.
Cited by
-
Effect of Saccharomyces cerevisiae ret1-1 mutation on glycosylation and localization of the secretome.Mol Cells. 2011 Feb;31(2):151-8. doi: 10.1007/s10059-011-0012-z. Epub 2010 Nov 25. Mol Cells. 2011. PMID: 21120625 Free PMC article.
-
Fluconazole Resistance Candida albicans in Females With Recurrent Vaginitis and Pir1 Overexpression.Jundishapur J Microbiol. 2015 Sep 23;8(9):e21468. doi: 10.5812/jjm.21468. eCollection 2015 Sep. Jundishapur J Microbiol. 2015. PMID: 26495107 Free PMC article.
-
Putative role of beta-1,3 glucans in Candida albicans biofilm resistance.Antimicrob Agents Chemother. 2007 Feb;51(2):510-20. doi: 10.1128/AAC.01056-06. Epub 2006 Nov 27. Antimicrob Agents Chemother. 2007. PMID: 17130296 Free PMC article.
-
Candida albicans ENO1 null mutants exhibit altered drug susceptibility, hyphal formation, and virulence.J Microbiol. 2013 Jun;51(3):345-51. doi: 10.1007/s12275-013-2577-z. Epub 2013 Jun 28. J Microbiol. 2013. PMID: 23812815
-
Assessment of the mechanism of drug resistance in Trichophyton mentagrophytes in response to various substances.BMC Genomics. 2021 Apr 7;22(1):250. doi: 10.1186/s12864-021-07520-6. BMC Genomics. 2021. PMID: 33827426 Free PMC article.
References
-
- Alloush, H. M., J. J. Lòpez-Ribot, B. J. Masten, and W. L. Chaffin. 1997. 3-Phosphoglycerate kinase: a glycolytic enzyme present in the cell wall of Candida albicans. Microbiology 143:321-330. - PubMed
-
- Angiolella, L., M. Facchin, A. Stringaro, B. Maras, N. Simonetti, and A. Cassone. 1996. Identification of a glucan-associated enolase as a main cell wall proteins of Candida albicans and an indirect target of lipopeptide antimycotics. J. Infect. Dis. 173:684-690. - PubMed
-
- Angiolella, L., N. Simonetti, and A. Cassone. 1994. The lipoptide antimycotic, cilofungin, modulates the incorporation of glucan-associated proteins into cell wall of Candida albicans. J. Antimicrob. Chemother. 33:1137-1146. - PubMed
-
- Cassone, A. 1986. Cell wall of pathogenic yeast and implications for antimycotic therapy. Drugs Exp. Clin. Res. 12:635-643. - PubMed
-
- Cassone, A., F. De Bernardis, E. Pontieri, G. Carruba, C. Girmenia, P. Martino, M. Fernández-Rodríguez, G. Quindós, and J. Ponton. 1995. Biotype diversità of Candida parapsilosis and its relationship to the clinical source and experimental pathogenicity. J. Infect. Dis. 171:967-975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

