Randomized controlled trial of sequential intravenous (i.v.) and oral moxifloxacin compared with sequential i.v. and oral co-amoxiclav with or without clarithromycin in patients with community-acquired pneumonia requiring initial parenteral treatment
- PMID: 12019085
- PMCID: PMC127227
- DOI: 10.1128/AAC.46.6.1746-1754.2002
Randomized controlled trial of sequential intravenous (i.v.) and oral moxifloxacin compared with sequential i.v. and oral co-amoxiclav with or without clarithromycin in patients with community-acquired pneumonia requiring initial parenteral treatment
Abstract
The objective of the present trial was to compare the efficacy, safety, and tolerability of moxifloxacin (400 mg) given intravenously (i.v.) once daily followed by oral moxifloxacin (400 mg) for 7 to 14 days with the efficacy, safety, and tolerability of co-amoxiclav (1.2 g) administered by i.v. infusion three times a day followed by oral co-amoxiclav (625 mg) three times a day, with or without clarithromycin (500 mg) twice daily (i.v. or orally), for 7 to 14 days in adult patients with community-acquired pneumonia requiring initial parenteral therapy. A total of 628 patients were enrolled and assessed by evaluation of their clinical and bacteriological responses 5 to 7 days and 21 to 28 days after administration of the last dose of study medication. Although the trial was designed, on the basis of predefined outcomes, to demonstrate the equivalence of the two regimens, the results showed statistically significant higher clinical success rates (for moxifloxacin, 93.4%, and for comparator regimen, 85.4%; difference [Delta], 8.05%; 95% confidence interval [CI], 2.91 to 13.19%; P = 0.004) and bacteriological success rates (for moxifloxacin, 93.7%, and for comparator regimen, 81.7%; Delta, 12.06%; 95% CI, 1.21 to 22.91%) for patients treated with moxifloxacin. This superiority was seen irrespective of the severity of the pneumonia and whether or not the combination therapy included a macrolide. The time to resolution of fever was also statistically significantly faster for patients who received moxifloxacin (median time, 2 versus 3 days), and the duration of hospital admission was approximately 1 day less for patients who received moxifloxacin. The treatment was converted to oral therapy immediately after the initial mandatory 3-day period of i.v. administration for a larger proportion of patients in the moxifloxacin group than patients in the comparator group (151 [50.2%] versus 57 [17.8%] patients). There were fewer deaths (9 [3.0%] versus 17 [5.3%]) and fewer serious adverse events (38 [12.6%] versus 53 [16.5%]) in the moxifloxacin group than in the comparator group. The rates of drug-related adverse events were comparable in both groups (38.9% in each treatment group). The overall incidence of laboratory abnormalities was similar in both groups. Thus, it is concluded that monotherapy with moxifloxacin is superior to that with a standard combination regimen of a beta-lactam and a beta-lactamase inhibitor, co-amoxiclav, with or without a macrolide, clarithromycin, in the treatment of patients with community-acquired pneumonia admitted to a hospital.
Figures

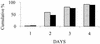
Comment in
-
Moxifloxacin for community-acquired pneumonia.Antimicrob Agents Chemother. 2003 Jan;47(1):444; author reply 444-5. doi: 10.1128/AAC.47.1.444-445.2003. Antimicrob Agents Chemother. 2003. PMID: 12499236 Free PMC article. No abstract available.
References
-
- Beam, T. R., D. N. Gilbert, and C. M. Kunin. 1992. General guidelines for the clinical evaluation of anti-infective drug products. Clin. Infect. Dis. 15(Suppl.):S5-S32. - PubMed
-
- Beam, T. R., D. N. Gilbert, and C. M. Kunin. 1993. General guidelines for the clinical evaluation of anti-infective drug products. European Society of Clinical Microbiology and Infectious Diseases, Munich, Germany.
-
- The British Thoracic Society. 1993. Guidelines for the management of community acquired pneumonia in adults admitted to hospital. Br. J. Hosp. Med. 49:346-350. - PubMed
-
- Committee for Proprietary Medicinal Products. 1997. Notes for guidance on evaluation of new anti-bacterial medical products. 3983:CPMP/EWP/558./95. The European Agency for the Evaluation of Medical Products, London, United Kingdom.
-
- Dresser, L. D., M. S. Niederman, and J. A. Paladino. 2001. Cost-effectiveness of gatifloxacin vs ceftriaxone with a macrolide for the treatment of community-acquired pneumonia. Chest 119:1439-1448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

