Double-blind, randomized, placebo-controlled study of topical 5% acyclovir-1% hydrocortisone cream (ME-609) for treatment of UV radiation-induced herpes labialis
- PMID: 12019102
- PMCID: PMC127265
- DOI: 10.1128/AAC.46.6.1870-1874.2002
Double-blind, randomized, placebo-controlled study of topical 5% acyclovir-1% hydrocortisone cream (ME-609) for treatment of UV radiation-induced herpes labialis
Abstract
Immunopathology is recognized as an important component of infectious disease manifestations, and corticosteroids have been used as an adjunct to antimicrobial therapy for a variety of conditions. Antiviral therapy of herpes labialis has been shown to result in only a small reduction in the time to healing and the duration of pain. To determine if topical application of a combination product containing 5% acyclovir and 1% hydrocortisone (ME-609) could provide benefit to herpes labialis patients, 380 immunocompetent adults with a history of herpes labialis were exposed to experimental UV radiation (UVR) to induce a recurrence. On day 2, just before the appearance of the majority of lesions ("delayed" lesions), subjects were randomized to receive active medication or vehicle control six times per day for 5 days. Overall, 120 of 380 patients developed delayed classical lesions, of whom 50 of 190 (26%) had been treated with ME-609 and 70 of 190 (37%) had received placebo (a reduction of 29% [P = 0.02]). Healing time, measured as the time to normal skin, was reduced by treatment with ME-609 (9.0 days for treated patients versus 10.1 days for the controls [P = 0.04]). There was a trend toward a reduction in the maximum lesion size in the ME-609 recipients compared to that in the controls (43 versus 60 mm(2), respectively [P = 0.07]). The treatment had no effect on lesion pain, but ME-609 treatment reduced the number of patients with moderate or severe tenderness. Compared to treatment with a placebo, treatment with the combination antiviral-immunomodulatory cream provided benefit to patients with experimental UVR-induced herpes labialis, reducing classical lesion incidence, healing time, lesion size, and lesion tenderness. ME-609 is a novel product that merits further evaluation as a treatment for cold sores.
Figures

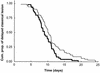

 ) is the time of observation of the first vesicle to the time of the first sign of crusting. The crusting stage (░⃞) is the time from the first sign of crusting to the time of the loss of the hard crust. The healing stage (□) is the time from the loss of the hard crust to normal skin.
) is the time of observation of the first vesicle to the time of the first sign of crusting. The crusting stage (░⃞) is the time from the first sign of crusting to the time of the loss of the hard crust. The healing stage (□) is the time from the loss of the hard crust to normal skin.Similar articles
-
Penciclovir cream for the treatment of herpes simplex labialis. A randomized, multicenter, double-blind, placebo-controlled trial. Topical Penciclovir Collaborative Study Group.JAMA. 1997 May 7;277(17):1374-9. JAMA. 1997. PMID: 9134943 Clinical Trial.
-
The role of topical 5% acyclovir and 1% hydrocortisone cream (Xerese™) in the treatment of recurrent herpes simplex labialis.Postgrad Med. 2010 Sep;122(5):1-6. doi: 10.3810/pgm.2010.09.2216. Postgrad Med. 2010. PMID: 20873400 Review.
-
Recent approval of Xerese in Canada: 5% acyclovir and 1% hydrocortisone topical cream in the treatment of herpes labialis.Skin Therapy Lett. 2014 May-Jun;19(3):5-8. Skin Therapy Lett. 2014. PMID: 25188362 Review.
-
Penciclovir cream for the treatment of sunlight-induced herpes simplex labialis: a randomized, double-blind, placebo-controlled trial. Penciclovir Cream Herpes Labialis Study Group.Clin Ther. 2000 Jan;22(1):76-90. doi: 10.1016/s0149-2918(00)87979-5. Clin Ther. 2000. PMID: 10688392 Clinical Trial.
-
Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials.Antimicrob Agents Chemother. 2002 Jul;46(7):2238-43. doi: 10.1128/AAC.46.7.2238-2243.2002. Antimicrob Agents Chemother. 2002. PMID: 12069980 Free PMC article. Clinical Trial.
Cited by
-
Current management and recommendations for access to antiviral therapy of herpes labialis.J Clin Virol. 2012 Jan;53(1):6-11. doi: 10.1016/j.jcv.2011.08.003. Epub 2011 Sep 1. J Clin Virol. 2012. PMID: 21889905 Free PMC article. Review.
-
Novel approaches in fighting herpes simplex virus infections.Expert Rev Anti Infect Ther. 2009 Jun;7(5):559-68. doi: 10.1586/eri.09.34. Expert Rev Anti Infect Ther. 2009. PMID: 19485796 Free PMC article. Review.
-
Herpes labialis.BMJ Clin Evid. 2009 Sep 23;2009:1704. BMJ Clin Evid. 2009. PMID: 21726482 Free PMC article.
-
Novel composite efficacy measure to demonstrate the rationale and efficacy of combination antiviral-anti-inflammatory treatment for recurrent herpes simplex labialis.Antimicrob Agents Chemother. 2014;58(3):1273-8. doi: 10.1128/AAC.02150-13. Epub 2013 Dec 16. Antimicrob Agents Chemother. 2014. PMID: 24342632 Free PMC article. Review.
-
Antiviral activity of Ferula assa-feotida on HSV-1, 2 in vitro.Iran J Microbiol. 2024 Dec;16(6):786-791. doi: 10.18502/ijm.v16i6.17257. Iran J Microbiol. 2024. PMID: 39737349 Free PMC article.
References
-
- Awan, A. R., J. Harmenberg, O. Flink, and H. J. Field. 1998. Combinations of antiviral and anti-inflammatory preparations for the topical treatment of herpes simplex virus assessed using a murine zosteriform infection model. Antivir. Chem. Chemother. 9:19-24. - PubMed
-
- Awan, A. R., J. Harmenberg, A. Kristofferson, and H. J. Field. 1998. Herpes simplex virus zosteriform lesions with adoptive transfer of immune cells: a murine model which mimics human recurrent disease. Antivir. Res. 38:43-53. - PubMed
-
- Raborn, G. W., W. T. McGaw, M. Grace, and J. Percy. 1988. Treatment of herpes labialis with acyclovir. Review of three clinical trials. Am. J. Med. 85:39-42. - PubMed
-
- Spruance, S. L. 1992. The natural history of recurrent oral-facial herpes simplex virus infection. Semin. Dermatol. 11:200-206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

