Quantitative assay for measuring the Taura syndrome virus and yellow head virus load in shrimp by real-time RT-PCR using SYBR Green chemistry
- PMID: 12020794
- PMCID: PMC7173102
- DOI: 10.1016/s0166-0934(02)00042-3
Quantitative assay for measuring the Taura syndrome virus and yellow head virus load in shrimp by real-time RT-PCR using SYBR Green chemistry
Abstract
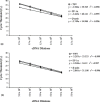
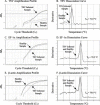
Taura syndrome virus (TSV) and yellow head virus (YHV) are the two RNA viruses infecting penaeid shrimp (Penaeus sp.) that have caused major economic losses to shrimp aquaculture. A rapid and highly sensitive detection and quantification method for TSV and YHV was developed using the GeneAmp 5700 Sequence Detection System and SYBR Green chemistry. The reverse transcriptase polymerase chain reaction (RT-PCR) mixture contained a fluorescent dye, SYBR Green, which exhibits fluorescence enhancement upon binding to double strand cDNA. The enhancement of fluorescence was found to be proportional to the initial concentration of the template cDNA. A linear relationship was observed between input plasmid DNA and cycle threshold (C(T)) values for 10(6) down to a single copy of both viruses. To control for the variation in sample processing and in reverse transcription reaction among samples, shrimp beta-actin and elongation factor-1alpha (EF-1alpha) genes were amplified in parallel with the viral cDNA. The sensitivity and the efficiency of amplification of EF-1alpha was greater than beta-actin when compared to TSV and YHV amplification efficiency suggesting that EF-1alpha is a better internal control for the RT-PCR detection of TSV and YHV. In addition, sample to sample variation in EF-1alpha C(T) value was lower than the variation in beta-actin C(T) value of the corresponding samples. The specificity of TSV, YHV, EF-1alpha and beta-actin amplifications was confirmed by analyzing the dissociation curves of the target amplicon. The C(T) values of TSV and YHV samples were normalized against EF-1alpha C(T) values for determining the absolute copy number from the standard curve of the corresponding virus. The method described here is highly robust and is amenable to high throughput assays making it a useful tool for diagnostic, epidemiological and genetic studies in shrimp aquaculture.
Figures


References
-
- Bonami J.R., Hasson K.W., Mari J., Poulos B.T., Lightner D.V. Taura syndrome of marine penaeid shrimp: characterization of the viral agent. J. Gen. Virol. 1997;78:313–319. - PubMed
-
- Brock J.A. Special topic review: Taura syndrome, a disease important to shrimp farms in the Americas. World J. Microbiol. Biotechnol. 1997;13:415–418.
-
- Brock, J.A., Gose, R., Lightner, D.V., Hasson, K.W., 1995. An overview of Taura syndrome, an important disease of farmed Penaeus vannamei. In: Browdy, C.L., Hopkin, J.S. (Eds.). Swimming through troubled water. Proceedings of the special session on shrimp farming. World Aquaculture Society, Baton Rouge, pp. 84–94.
-
- Bustin S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000;25:169–193. - PubMed
-
- Chantanachookin C., Boonyaratpalin S., Kasornchandra J., Direkbusarakom S., Ekpanithanpong U., Supamataya K., Sriurairatana S., Flegel T.W. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by yellow-head disease. Dis. Aquat. Org. 1993;17:145–157.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

