Whence nitrotyrosine?
- PMID: 12021243
- PMCID: PMC150989
- DOI: 10.1172/JCI15816
Whence nitrotyrosine?
Figures
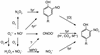
Comment on
-
Myeloperoxidase produces nitrating oxidants in vivo.J Clin Invest. 2002 May;109(10):1311-9. doi: 10.1172/JCI15021. J Clin Invest. 2002. PMID: 12021246 Free PMC article.
References
-
- Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. - PubMed
-
- Jourd’heuil D, et al. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J Biol Chem. 2001;276:28799–28805. - PubMed
-
- Goldstein S, Czapski G, Lind J, Merényi G. Tyrosine nitration by simultaneous generation of •NO and O2•-under physiological conditions. How the radicals do the job. J Biol Chem. 2000;275:3031–3036. - PubMed
-
- Lymar SV, Jiang Q, Hurst JK. Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry. 1996;35:7855–7861. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

