Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins
- PMID: 12021245
- PMCID: PMC2447671
- DOI: 10.1172/JCI14506
Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins
Abstract
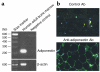
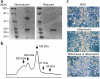
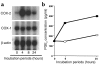
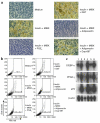
Adiponectin, an adipocyte-derived hormone, was recently shown to have potential therapeutic applications in diabetes and obesity because of its influence on glucose and lipid metabolism. We found that brown fat in normal human bone marrow contains this protein and used marrow-derived preadipocyte lines and long-term cultures to explore potential roles in hematopoiesis. Recombinant adiponectin blocked fat cell formation in long-term bone marrow cultures and inhibited the differentiation of cloned stromal preadipocytes. Adiponectin also caused elevated expression of cyclooxygenase-2 (COX-2) by these stromal cells and induced release of prostaglandin E(2) (PGE(2)). The COX-2 inhibitor Dup-697 prevented the inhibitory action of adiponectin on preadipocyte differentiation, suggesting involvement of stromal cell-derived prostanoids. Furthermore, adiponectin failed to block fat cell generation when bone marrow cells were derived from B6,129S(Ptgs2tm1Jed) (COX-2(+/-)) mice. These observations show that preadipocytes represent direct targets for adiponectin action, establishing a paracrine negative feedback loop for fat regulation. They also link adiponectin to the COX-2-dependent PGs that are critical in this process.
Figures

References
-
- Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Gimble JM, et al. Adipogenesis in a murine bone marrow stromal cell line capable of supporting B lineage lymphocyte growth and proliferation: biochemical and molecular characterization. Eur J Immunol. 1990;20:379–387. - PubMed
-
- Forman BM, et al. 15-deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. - PubMed
-
- Kliewer SA, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

