Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration
- PMID: 12021255
- PMCID: PMC2173424
- DOI: 10.1083/jcb.200108150
Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration
Abstract
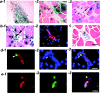
Three populations of myogenic cells were isolated from normal mouse skeletal muscle based on their adhesion characteristics and proliferation behaviors. Although two of these populations displayed satellite cell characteristics, a third population of long-time proliferating cells expressing hematopoietic stem cell markers was also identified. This third population comprises cells that retain their phenotype for more than 30 passages with normal karyotype and can differentiate into muscle, neural, and endothelial lineages both in vitro and in vivo. In contrast to the other two populations of myogenic cells, the transplantation of the long-time proliferating cells improved the efficiency of muscle regeneration and dystrophin delivery to dystrophic muscle. The long-time proliferating cells' ability to proliferate in vivo for an extended period of time, combined with their strong capacity for self-renewal, their multipotent differentiation, and their immune-privileged behavior, reveals, at least in part, the basis for the improvement of cell transplantation. Our results suggest that this novel population of muscle-derived stem cells will significantly improve muscle cell-mediated therapies.
Figures





References
-
- Baroffio, A., M. Hamann, L. Bernheim, M.L. Bochaton-Piallat, G. Gabbiani, and C.R. Bader. 1996. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 60:47–57. - PubMed
-
- Barch, M.J. 1991. Chromosome analysis. The ACT Cytogenetics Laboratory Manual. 2nd ed. Raven Press, Ltd., New York. 349.
-
- Bischoff, R. 1986. Proliferation of muscle satellite cells on intact myofibers in culture. Dev. Biol. 115:129–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

