Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells
- PMID: 12021308
- PMCID: PMC2193752
- DOI: 10.1084/jem.20012100
Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells
Erratum in
- J Exp Med. 2003 Feb 3;197(3):395.
Abstract
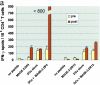
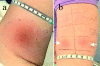
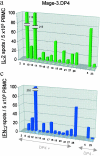
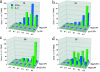
There is consensus that an optimized cancer vaccine will have to induce not only CD8+ cytotoxic but also CD4+ T helper (Th) cells, particularly interferon (IFN)-gamma-producing, type 1 Th cells. The induction of strong, ex vivo detectable type 1 Th cell responses has not been reported to date. We demonstrate now that the subcutaneous injection of cryopreserved, mature, antigen-loaded, monocyte-derived dendritic cells (DCs) rapidly induces unequivocal Th1 responses (ex vivo detectable IFN-gamma-producing effectors as well as proliferating precursors) both to the control antigen KLH and to major histocompatibility complex (MHC) class II-restricted tumor peptides (melanoma-antigen [Mage]-3.DP4 and Mage-3.DR13) in the majority of 16 evaluable patients with metastatic melanoma. These Th1 cells recognized not only peptides, but also DCs loaded with Mage-3 protein, and in case of Mage-3DP4-specific Th1 cells IFN-gamma was released even after direct recognition of viable, Mage-3-expressing HLA-DP4+ melanoma cells. The capacity of DCs to rapidly induce Th1 cells should be valuable to evaluate whether Th1 cells are instrumental in targeting human cancer and chronic infections.
Figures





References
-
- Boon, T., P.G. Coulie, and B. van den Eynde. 1997. Tumor antigens recognized by T cells. Immunol. Today. 18:267–268. - PubMed
-
- Rosenberg, S.A. 1999. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 10:281–287. - PubMed
-
- Pardoll, D.M., and S.L. Topalian. 1998. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 10:588–594. - PubMed
-
- Wang, R. 2001. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 22:269–276. - PubMed
-
- Ada, G. 2001. Vaccines and vaccination. N. Engl. J. Med. 345:1042–1053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

