CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis
- PMID: 12021312
- PMCID: PMC2193749
- DOI: 10.1084/jem.20011565
CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis
Abstract
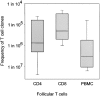
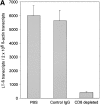
The assembly of inflammatory lesions in rheumatoid arthritis is highly regulated and typically leads to the formation of lymphoid follicles with germinal center (GC) reactions. We used microdissection of such extranodal follicles to analyze the colonizing T cells. Although the repertoire of follicular T cells was diverse, a subset of T cell receptor (TCR) sequences was detected in multiple independent follicles and not in interfollicular zones, suggesting recognition of a common antigen. Unexpectedly, the majority of shared TCR sequences were from CD8 T cells that were highly enriched in the synovium and present in low numbers in the periphery. To examine their role in extranodal GC reactions, CD8 T cells were depleted in human synovium-SCID mouse chimeras. Depletion of synovial CD8 T cells caused disintegration of the GC-containing follicles. In the absence of CD8 T cells, follicular dendritic cells disappeared, production of lymphotoxin-alpha1beta2 markedly decreased, and immunoglobulin (Ig) secretion ceased. Immunohistochemical studies demonstrated that these CD8 T cells accumulated at the edge of the mantle zone. Besides their unique localization, they were characterized by the production of interferon (IFN)-gamma, lack of the pore-forming enzyme perforin, and expression of CD40 ligand. Perifollicular IFN-gamma+ CD8 T cells were rare in secondary lymphoid tissues but accounted for the majority of IFN-gamma+ cells in synovial infiltrates. We propose that CD8+ T cells regulate the structural integrity and functional activity of GCs in ectopic lymphoid follicles.
Figures














Similar articles
-
Ectopic germinal center formation in rheumatoid synovitis.Ann N Y Acad Sci. 2003 Apr;987:140-9. doi: 10.1111/j.1749-6632.2003.tb06042.x. Ann N Y Acad Sci. 2003. PMID: 12727633 Review.
-
The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis.J Immunol. 1998 Dec 1;161(11):6390-7. J Immunol. 1998. PMID: 9834130
-
IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis.J Immunol. 1999 Apr 1;162(7):4293-9. J Immunol. 1999. PMID: 10201961
-
T cell activation in rheumatoid synovium is B cell dependent.J Immunol. 2001 Oct 15;167(8):4710-8. doi: 10.4049/jimmunol.167.8.4710. J Immunol. 2001. PMID: 11591802
-
Morphological and molecular pathology of the B cell response in synovitis of rheumatoid arthritis.Virchows Arch. 2002 Nov;441(5):415-27. doi: 10.1007/s00428-002-0702-1. Epub 2002 Nov 5. Virchows Arch. 2002. PMID: 12447670 Review.
Cited by
-
Potential of Cells and Cytokines/Chemokines to Regulate Tertiary Lymphoid Structures in Human Diseases.Immune Netw. 2016 Oct;16(5):271-280. doi: 10.4110/in.2016.16.5.271. Epub 2016 Oct 25. Immune Netw. 2016. PMID: 27799872 Free PMC article. Review.
-
Failure of catecholamines to shift T-cell cytokine responses toward a Th2 profile in patients with rheumatoid arthritis.Arthritis Res Ther. 2006;8(5):R138. doi: 10.1186/ar2028. Arthritis Res Ther. 2006. PMID: 16889669 Free PMC article.
-
T cell help in the autoreactive germinal center.Scand J Immunol. 2022 Jun;95(6):e13192. doi: 10.1111/sji.13192. Epub 2022 May 31. Scand J Immunol. 2022. PMID: 35587582 Free PMC article. Review.
-
Lymphoid neogenesis and immune infiltration in aged liver.Hepatology. 2008 May;47(5):1680-90. doi: 10.1002/hep.22224. Hepatology. 2008. PMID: 18395842 Free PMC article.
-
Promise, Progress, and Pitfalls in the Search for Central Nervous System Biomarkers in Neuroimmunological Diseases: A Role for Cerebrospinal Fluid Immunophenotyping.Semin Pediatr Neurol. 2017 Aug;24(3):229-239. doi: 10.1016/j.spen.2017.08.001. Epub 2017 Aug 12. Semin Pediatr Neurol. 2017. PMID: 29103430 Free PMC article. Review.
References
-
- Nepom, G.T. 1998. Major histocompatibility complex-directed susceptibility to rheumatoid arthritis. Adv. Immunol. 68:315–332. - PubMed
-
- Young, C.L., T.C. Adamson, J.H. Vaughan, and R.I. Fox. 1984. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthr. Rheum. 27:32–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

